Control of cell-cell forces and collective cell dynamics by the intercellular adhesome
- PMID: 25812522
- PMCID: PMC4886824
- DOI: 10.1038/ncb3135
Control of cell-cell forces and collective cell dynamics by the intercellular adhesome
Abstract
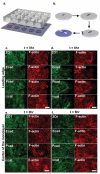
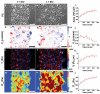
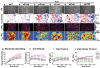
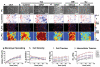
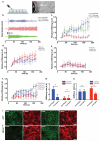
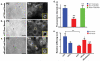
Dynamics of epithelial tissues determine key processes in development, tissue healing and cancer invasion. These processes are critically influenced by cell-cell adhesion forces. However, the identity of the proteins that resist and transmit forces at cell-cell junctions remains unclear, and how these proteins control tissue dynamics is largely unknown. Here we provide a systematic study of the interplay between cell-cell adhesion proteins, intercellular forces and epithelial tissue dynamics. We show that collective cellular responses to selective perturbations of the intercellular adhesome conform to three mechanical phenotypes. These phenotypes are controlled by different molecular modules and characterized by distinct relationships between cellular kinematics and intercellular forces. We show that these forces and their rates can be predicted by the concentrations of cadherins and catenins. Unexpectedly, we identified different mechanical roles for P-cadherin and E-cadherin; whereas P-cadherin predicts levels of intercellular force, E-cadherin predicts the rate at which intercellular force builds up.
Figures


References
-
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nature reviews. Molecular cell biology. 2009;10:445–457. - PubMed
-
- Heisenberg CP, Bellaiche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. - PubMed
-
- Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Current opinion in cell biology. 2011;23:523–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

