Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis
- PMID: 25812968
- PMCID: PMC4756280
- DOI: 10.1016/S2213-2600(15)00095-8
Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis
Abstract
Background: Microbiological confirmation of childhood tuberculosis is rare because of the difficulty of collection of specimens, low sensitivity of smear microscopy, and poor access to culture. We aimed to establish summary estimates for sensitivity and specificity of of the Xpert MTB/RIF assay compared with microscopy in the diagnosis of pulmonary tuberculosis in children.
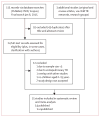
Methods: We searched for studies published up to Jan 6, 2015, that used Xpert in any setting in children with and without HIV infection. We systematically reviewed studies that compared the diagnostic accuracy of Xpert MTB/RIF (Xpert) with microscopy for detection of pulmonary tuberculosis and rifampicin resistance in children younger than 16 years against two reference standards-culture results and culture-negative children who were started on anti-tuberculosis therapy. We did meta-analyses using a bivariate random-effects model.
Findings: We identified 15 studies including 4768 respiratory specimens in 3640 children investigated for pulmonary tuberculosis. Culture tests were positive for tuberculosis in 12% (420 of 3640) of all children assessed and Xpert was positive in 11% (406 of 3640). Compared with culture, the pooled sensitivities and specificities of Xpert for tuberculosis detection were 62% (95% credible interval 51-73) and 98% (97-99), respectively, with use of expectorated or induced sputum samples and 66% (51-81) and 98% (96-99), respectively, with use of samples from gastric lavage. Xpert sensitivity was 36-44% higher than was sensitivity for microscopy. Xpert sensitivity in culture-negative children started on antituberculosis therapy was 2% (1-3) for expectorated or induced sputum. Xpert's pooled sensitivity and specificity to detect rifampicin resistance was 86% (95% credible interval 53-98) and 98% (94-100), respectively.
Interpretation: Compared with microscopy, Xpert offers better sensitivity for the diagnosis of pulmonary tuberculosis in children and its scale-up will improve access to tuberculosis diagnostics for children. Although Xpert helps to provide rapid confirmation of disease, its sensitivity remains suboptimum compared with culture tests. A negative Xpert result does not rule out tuberculosis. Good clinical acumen is still needed to decide when to start antituberculosis therapy and continued research for better diagnostics is crucial.
Funding: WHO, Global TB Program of Texas Children's Hospital.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare no competing interests.
Figures



Comment in
-
Xpert MTB/RIF to diagnose tuberculosis in children.Lancet Respir Med. 2015 Jun;3(6):419-21. doi: 10.1016/S2213-2600(15)00111-3. Epub 2015 Mar 24. Lancet Respir Med. 2015. PMID: 25812969 No abstract available.
References
-
- WHO. Global Tuberculosis Report. Geneva: World Health Organization; 2014.
-
- Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–59. - PubMed
-
- Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics. 2006;118:e1350–59. - PubMed
-
- WHO. [accessed March 15, 2015];Policy update: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. 2013 http://www.who.int/tb/laboratory/xpert_launchupdate/en/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

