Wnt5a and Wnt11 regulate mammalian anterior-posterior axis elongation
- PMID: 25813538
- PMCID: PMC4392599
- DOI: 10.1242/dev.119065
Wnt5a and Wnt11 regulate mammalian anterior-posterior axis elongation
Abstract
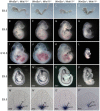
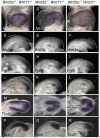
Mesoderm formation and subsequent anterior-posterior (A-P) axis elongation are fundamental aspects of gastrulation, which is initiated by formation of the primitive streak (PS). Convergent extension (CE) movements and epithelial-mesenchymal transition (EMT) are important for A-P axis elongation in vertebrate embryos. The evolutionarily conserved planar cell polarity (PCP) pathway regulates CE, and Wnts regulate many aspects of gastrulation including CE and EMT. However, the Wnt ligands that regulate A-P axis elongation in mammalian development remain unknown. Wnt11 and Wnt5a regulate axis elongation in lower vertebrates, but only Wnt5a, not Wnt11, regulates mammalian PCP signaling and A-P axis elongation in development. Here, by generating Wnt5a; Wnt11 compound mutants, we show that Wnt11 and Wnt5a play redundant roles during mouse A-P axis elongation. Both genes regulate trunk notochord extension through PCP-controlled CE of notochord cells, establishing a role for Wnt11 in mammalian PCP. We show that Wnt5a and Wnt11 are required for proper patterning of the neural tube and somites by regulating notochord formation, and provide evidence that both genes are required for the generation and migration of axial and paraxial mesodermal precursor cells by regulating EMT. Axial and paraxial mesodermal precursors ectopically accumulate in the PS at late gastrula stages in Wnt5a(-/-); Wnt11(-/-) embryos and these cells ectopically express epithelial cell adhesion molecules. Our data suggest that Wnt5a and Wnt11 regulate EMT by inducing p38 (Mapk14) phosphorylation. Our findings provide new insights into the role of Wnt5a and Wnt11 in mouse early development and also in cancer metastasis, during which EMT plays a crucial role.
Keywords: Convergent extension; EMT; Gastrulation; Notochord; Planar cell polarity; Wnt.
© 2015. Published by The Company of Biologists Ltd.
Figures





References
-
- Abdelkhalek H. B., Beckers A., Schuster-Gossler K., Pavlova M. N., Burkhardt H., Lickert H., Rossant J., Reinhardt R., Schalkwyk L. C., Müller I. et al. (2004). The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 18, 1725-1736 10.1101/gad.303504 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

