The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis
- PMID: 25814158
- PMCID: PMC4814953
- DOI: 10.4103/1008-682X.150844
The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis
Abstract
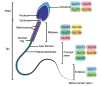
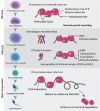
The effects of diabetes mellitus include long-term damages, dysfunctions, and failures of various organs. An important complication of diabetes is the disturbance in the male reproductive system. Glucose metabolism is an important event in spermatogenesis. Moreover, glucose metabolism is also important for maintaining basic cell activity, as well as specific functions, such as motility and fertilization ability in mature sperm. Diabetic disease and experimentally induced diabetes both demonstrated that either type 1 diabetes or type 2 diabetes could have detrimental effects on male fertility, especially on sperm quality, such as sperm motility, sperm DNA integrity, and ingredients of seminal plasma. Epigenetic modifications are essential during spermatogenesis. The epigenetic regulation represents chromatin modifications including DNA methylation, histone modifications, remodeling of nucleosomes and the higher-order chromatin reorganization and noncoding RNAs. If spermatogenesis is affected during the critical developmental window, embryonic gonadal development, and germline differentiation, environmentally-induced epigenetic modifications may become permanent in the germ line epigenome and have a potential impact on subsequent generations through epigenetic transgenerational inheritance. Diabetes may influence the epigenetic modification during sperm spermatogenesis and that these epigenetic dysregulation may be inherited through the male germ line and passed onto more than one generation, which in turn may increase the risk of diabetes in offspring.
Figures
References
-
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. - PubMed
-
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. - PubMed
-
- Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–26. - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. - PubMed
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

