Cofilin-2 phosphorylation and sequestration in myocardial aggregates: novel pathogenetic mechanisms for idiopathic dilated cardiomyopathy
- PMID: 25814227
- PMCID: PMC4379451
- DOI: 10.1016/j.jacc.2015.01.031
Cofilin-2 phosphorylation and sequestration in myocardial aggregates: novel pathogenetic mechanisms for idiopathic dilated cardiomyopathy
Erratum in
- J Am Coll Cardiol. 2015 May 12;65(18):2056
Abstract
Background: Recently, tangles and plaque-like aggregates have been identified in certain cases of dilated cardiomyopathy (DCM), traditionally labeled idiopathic (iDCM), where there is no specific diagnostic test or targeted therapy. This suggests a potential underlying cause for some of the iDCM cases. [Corrected]
Objectives: This study sought to identify the make-up of myocardial aggregates to understand the molecular mechanisms of these cases of DCM; this strategy has been central to understanding Alzheimer's disease.
Methods: Aggregates were extracted from human iDCM samples with high congophilic reactivity (an indication of plaque presence), and the findings were validated in a larger cohort of samples. We tested the expression, distribution, and activity of cofilin in human tissue and generated a cardiac-specific knockout mouse model to investigate the functional impact of the human findings. We also modeled cofilin inactivity in vitro by using pharmacological and genetic gain- and loss-of-function approaches.
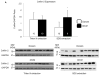
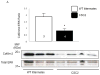
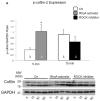
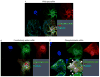
Results: Aggregates in human myocardium were enriched for cofilin-2, an actin-depolymerizing protein known to participate in neurodegenerative diseases and nemaline myopathy. Cofilin-2 was predominantly phosphorylated, rendering it inactive. Cardiac-specific haploinsufficiency of cofilin-2 in mice recapitulated the human disease's morphological, functional, and structural phenotype. Pharmacological stimulation of cofilin-2 phosphorylation and genetic overexpression of the phosphomimetic protein promoted the accumulation of "stress-like" fibers and severely impaired cardiomyocyte contractility.
Conclusions: Our study provides the first biochemical characterization of prefibrillar myocardial aggregates in humans and the first report to link cofilin-2 to cardiomyopathy. The findings suggest a common pathogenetic mechanism connecting certain iDCMs and other chronic degenerative diseases, laying the groundwork for new therapeutic strategies.
Keywords: adenovirus; heart failure; nemaline.
Copyright © 2015 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures










Comment in
-
Entangled in a heart-ailing quandary: could modified cofilin-2 be a culprit of Alzheimer's disease of the heart?J Am Coll Cardiol. 2015 Mar 31;65(12):1215-1217. doi: 10.1016/j.jacc.2015.01.035. J Am Coll Cardiol. 2015. PMID: 25814228 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

