The Effect of Bi-Terminal PEGylation of an Integrin αvβ₆-Targeted ¹⁸F Peptide on Pharmacokinetics and Tumor Uptake
- PMID: 25814519
- PMCID: PMC4559355
- DOI: 10.2967/jnumed.114.150680
The Effect of Bi-Terminal PEGylation of an Integrin αvβ₆-Targeted ¹⁸F Peptide on Pharmacokinetics and Tumor Uptake
Abstract
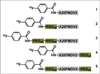
Radiotracers based on the peptide A20FMDV2 selectively target the cell surface receptor integrin αvβ6. This integrin has been identified as a prognostic indicator correlating with the severity of disease for several challenging malignancies. In previous studies of A20FMDV2 peptides labeled with 4-(18)F-fluorobenzoic acid ((18)F-FBA), we have shown that the introduction of poly(ethylene glycol) (PEG) improves pharmacokinetics, including increased uptake in αvβ6-expressing tumors. The present study evaluated the effect of site-specific C-terminal or dual (N- and C-terminal) PEGylation, yielding (18)F-FBA-A20FMDV2-PEG28 (4) and (18)F-FBA-PEG28-A20FMDV2-PEG28 (5), on αvβ6-targeted tumor uptake and pharmacokinetics. The results are compared with (18)F-FBA -labeled A20FMDV2 radiotracers (1- 3) bearing either no PEG or different PEG units at the N terminus.
Methods: The radiotracers were prepared and radiolabeled on solid phase. Using 3 cell lines, DX3puroβ6 (αvβ6+), DX3puro (αvβ6-), and BxPC-3 (αvβ6+), we evaluated the radiotracers in vitro (serum stability; cell binding and internalization) and in vivo in mouse models bearing paired DX3puroβ6-DX3puro and, for 5, BxPC-3 xenografts.
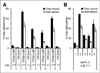
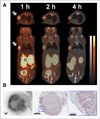
Results: The size and location of the PEG units significantly affected αvβ6 targeting and pharmacokinetics. Although the C-terminally PEGylated 4 showed some improvements over the un-PEGylated (18)F-FBA-A20FMDV2 (1), it was the bi-terminally PEGylated 5 that displayed the more favorable combination of high αvβ6 affinity, selectivity, and pharmacokinetic profile. In vitro, 5 bound to αvβ6-expressing DX3puroβ6 and BxPC-3 cells with 60.5% ± 3.3% and 48.8% ± 8.3%, respectively, with a significant fraction of internalization (37.2% ± 4.0% and 37.6% ± 4.1% of total radioactivity, respectively). By comparison, in the DX3puro control 5: showed only 3.0% ± 0.5% binding and 0.9% ± 0.2% internalization. In vivo, 5: maintained high, αvβ6-directed binding in the paired DX3puroβ6-DX3puro model (1 h: DX3puroβ6, 2.3 ± 0.2 percentage injected dose per gram [%ID/g]; DX3puroβ6/DX3puro ratio, 6.5:1; 4 h: 10.7:1). In the pancreatic BxPC-3 model, uptake was 4.7 ± 0.9 %ID/g (1 h) despite small tumor sizes (20-80 mg).
Conclusion: The bi-PEGylated radiotracer 5 showed a greatly improved pharmacokinetic profile, beyond what was predicted from individual N- or C-terminal PEGylation. It appears that the 2 PEG units acted synergistically to result in an improved metabolic profile including high αvβ6+ tumor uptake and retention.
Keywords: PEGylation; integrin αvβ6; metabolism; peptide; positron emission tomography.
© 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures


References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Srichai MN, Zent R. Integrin structure and function. In: Zent R, Pozzi A, editors. Cell-Extracellular Matrix Interactions in Cancer. New York, NY: Springer; 2010. pp. 19–41.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
