CFH Y402H polymorphism is associated with elevated vitreal GM-CSF and choroidal macrophages in the postmortem human eye
- PMID: 25814824
- PMCID: PMC4360164
CFH Y402H polymorphism is associated with elevated vitreal GM-CSF and choroidal macrophages in the postmortem human eye
Abstract
Purpose: Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in people 50 years of age or older in developed countries. The homozygous CC genotype in the complement factor H (CFH) Y402H single nucleotide polymorphism (SNP; rs1061170) is widely recognized as a risk factor for the development of AMD. In this study, we examined vitreal levels of granulocyte macrophage colony-stimulating factor (GM-CSF), a hematopoietic cytokine, and macrophages in the choroid of postmortem human eyes genotyped for the CFH Y402H SNP.
Methods: Twenty-two pairs of postmortem, non-diseased, human donor eyes were obtained. The vitreous and retinal tissues of the left eyes were collected for GM-CSF level measurement and CFH Y402H genotyping, respectively. The right eyes were paraffin-embedded and sectioned for immunohistochemistry using a macrophage and microglia marker, CD68. Cell cultures of RPE cells were stimulated with complement C3a, C5a, 4-hydroxynonenal (HNE), or tumor necrosis factor alpha (TNF-α), and GM-CSF expression was measured with a suspension assay or quantitative PCR.
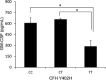
Results: Eyes genotyped with the CC or the CT risk variant of the CFH Y402H SNP showed significantly increased levels of GM-CSF in the vitreous compared to eyes with the protective TT variant (mean ± standard error of mean, 607.54±85.83 pg/ml or 656.32±15.20 pg/ml versus 286.69±81.96 pg/ml, p<0.05). The choroid of eye tissues genotyped with the CC variant showed higher levels of CD68 immunoreactivity than the tissues genotyped with the TT variant (p<0.05). The GM-CSF levels detected in the supernatant of RPE cells in culture treated with HNE or TNF-α were significantly higher compared to the non-treated control (145.88±5.06 pg/ml and 149.32±3.76 pg/ml versus 123.27±4.05 pg/ml, p<0.05). Furthermore, the gene expression of GM-CSF detected in the lysate of RPE cells stimulated with complement C3a or C5a showed significantly increased fold changes compared to the non-treated control (C3a: 2.38±0.31 fold, p<0.05; C5a: 2.84±0.54 fold, p<0.01).
Conclusions: Our data showed a relationship between the CFH Y402H polymorphism and GM-CSF levels in the vitreous and accumulation of choroidal macrophages in the postmortem eye. These data suggest that the at-risk variant of the CFH gene may contribute to the dysregulation of proinflammatory cytokines locally in the eye.
Figures



References
-
- Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Eye Diseases Prevalence Research G. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. - PubMed
-
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–17. - PubMed
-
- Khandhadia S, Cherry J, Lotery AJ. Age-related macular degeneration. Adv Exp Med Biol. 2012;724:15–36. - PubMed
-
- Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya'ale D, Negrel AD, Resnikoff S. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11:67–115. - PubMed
-
- Kaarniranta K, Salminen A, Haapasalo A, Soininen H, Hiltunen M. Age-related macular degeneration (AMD): Alzheimer's disease in the eye? J Alzheimers Dis. 2011;24:615–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

