Plasma metabolomics reveals a potential panel of biomarkers for early diagnosis in acute coronary syndrome
- PMID: 25814918
- PMCID: PMC4363481
- DOI: 10.1007/s11306-013-0595-9
Plasma metabolomics reveals a potential panel of biomarkers for early diagnosis in acute coronary syndrome
Abstract
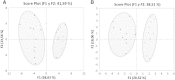
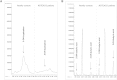
Discovery of new biomarkers is critical for early diagnosis of acute coronary syndrome (ACS). Recent advances in metabolomic technologies have drastically enhanced the possibility of improving the knowledge of its physiopathology through the identification of the altered metabolic pathways. In this study, analyses of peripheral plasma from non-ST segment elevation ACS patients and healthy controls by gas chromatography-mass spectrometry (GC-MC) permitted the identification of 15 metabolites with statistical differences (p < 0.05) between experimental groups. Additionally, validation by GC-MC and liquid chromatography-MC permitted us to identify a potential panel of biomarkers formed by 5-OH-tryptophan, 2-OH-butyric acid and 3-OH-butyric acid. This panel of biomarkers reflects the oxidative stress and the hypoxic state that suffers the myocardial cells and consequently constitutes a metabolomic signature of the atherogenesis process that could be used for early diagnosis of ACS.
Keywords: Acute coronary syndrome; Biomarker; Metabolite; Metabolomics.
Figures


Similar articles
-
A Comparative Metabolomics Approach Reveals Early Biomarkers for Metabolic Response to Acute Myocardial Infarction.Sci Rep. 2016 Nov 8;6:36359. doi: 10.1038/srep36359. Sci Rep. 2016. PMID: 27821850 Free PMC article.
-
The plasma proteomic signature as a strategic tool for early diagnosis of acute coronary syndrome.Proteome Sci. 2014 Oct 10;12:43. doi: 10.1186/1477-5956-12-43. eCollection 2014. Proteome Sci. 2014. PMID: 26038678 Free PMC article.
-
Urinary metabolomics analysis based on LC-MS for the diagnosis and monitoring of acute coronary syndrome.Front Mol Biosci. 2025 Apr 9;12:1547476. doi: 10.3389/fmolb.2025.1547476. eCollection 2025. Front Mol Biosci. 2025. PMID: 40270590 Free PMC article.
-
Metabolomics in early detection and prognosis of acute coronary syndrome.Clin Chim Acta. 2019 Aug;495:43-53. doi: 10.1016/j.cca.2019.03.1632. Epub 2019 Mar 27. Clin Chim Acta. 2019. PMID: 30928571 Review.
-
Recent progress in metabolomic analysis of acute coronary syndrome: a narrative review.Cardiovasc Diagn Ther. 2025 Apr 30;15(2):480-499. doi: 10.21037/cdt-24-431. Epub 2025 Apr 23. Cardiovasc Diagn Ther. 2025. PMID: 40385284 Free PMC article. Review.
Cited by
-
Metabolomics and partial least square discriminant analysis to predict history of myocardial infarction of self-claimed healthy subjects: validity and feasibility for clinical practice.J Clin Bioinforma. 2015 Mar 13;5:3. doi: 10.1186/s13336-015-0018-4. eCollection 2015. J Clin Bioinforma. 2015. PMID: 25806102 Free PMC article.
-
A Comparative Metabolomics Approach Reveals Early Biomarkers for Metabolic Response to Acute Myocardial Infarction.Sci Rep. 2016 Nov 8;6:36359. doi: 10.1038/srep36359. Sci Rep. 2016. PMID: 27821850 Free PMC article.
-
KLK1 and ZG16B proteins and arginine-proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery.Metabolomics. 2015;11(5):1056-1067. doi: 10.1007/s11306-014-0761-8. Epub 2014 Dec 14. Metabolomics. 2015. PMID: 26413039 Free PMC article.
-
Innovative Approaches to Assess Intermediate Cardiovascular Risk Subjects: A Review From Clinical to Metabolomics Strategies.Front Cardiovasc Med. 2021 Dec 22;8:788062. doi: 10.3389/fcvm.2021.788062. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 35004898 Free PMC article. Review.
-
Defining Acute Coronary Syndrome through Metabolomics.Metabolites. 2021 Oct 6;11(10):685. doi: 10.3390/metabo11100685. Metabolites. 2021. PMID: 34677400 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
