Molecular cloning and characterization of the allatotropin precursor and receptor in the desert locust, Schistocerca gregaria
- PMID: 25814925
- PMCID: PMC4357254
- DOI: 10.3389/fnins.2015.00084
Molecular cloning and characterization of the allatotropin precursor and receptor in the desert locust, Schistocerca gregaria
Abstract
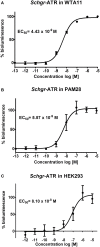
Allatotropins (ATs) are pleiotropic neuropeptides initially isolated from the tobacco hornworm, Manduca sexta. In 2008, the first receptor for AT-like peptides (ATR) was characterized in Bombyx mori. Since then, ATRs have also been characterized in M. sexta, Tribolium castaneum, Aedes aegypti and Bombus terrestris. These receptors show sequence similarity to vertebrate orexin (ORX) receptors. When generating an EST-database of the desert locust (Schistocerca gregaria) central nervous system, we found cDNA sequences encoding the Schgr-AT precursor and a fragment of its putative receptor. This receptor cDNA has now been completed and functionally expressed in mammalian cell lines. Activation of this receptor, designated as Schgr-ATR, by Schgr-AT caused an increase in intracellular calcium ions, as well as cyclic AMP (cAMP), with an EC50 value in the nanomolar range. In addition, the transcript distribution of both the Schgr-AT precursor and Schgr-ATR was investigated by means of quantitative real-time PCR. Moreover, we found more evidence for the myotropic and allatostimulatory actions of Schgr-AT in the desert locust. These data are discussed and situated in a broader context by comparison with literature data on AT and ATR in insects.
Keywords: GPCR; insect; juvenile hormone; motility; neuropeptide; orexin; peptide.
Figures





Similar articles
-
Characterisation of a functional allatotropin receptor in the bumblebee, Bombus terrestris (Hymenoptera, Apidae).Gen Comp Endocrinol. 2013 Nov 1;193:193-200. doi: 10.1016/j.ygcen.2013.08.006. Epub 2013 Aug 19. Gen Comp Endocrinol. 2013. PMID: 23968772
-
Isolation and functional characterization of an allatotropin receptor from Manduca sexta.Insect Biochem Mol Biol. 2011 Oct;41(10):804-14. doi: 10.1016/j.ibmb.2011.06.002. Epub 2011 Jun 14. Insect Biochem Mol Biol. 2011. PMID: 21699978
-
Identification of the short neuropeptide F precursor in the desert locust: evidence for an inhibitory role of sNPF in the control of feeding.Peptides. 2014 Mar;53:134-9. doi: 10.1016/j.peptides.2013.09.018. Epub 2013 Oct 12. Peptides. 2014. PMID: 24128610
-
Peptides in the locusts, Locusta migratoria and Schistocerca gregaria.Peptides. 1997;18(1):145-56. doi: 10.1016/s0196-9781(96)00236-7. Peptides. 1997. PMID: 9114464 Review.
-
The Intricate Role of Ecdysis Triggering Hormone Signaling in Insect Development and Reproductive Regulation.Insects. 2023 Aug 16;14(8):711. doi: 10.3390/insects14080711. Insects. 2023. PMID: 37623421 Free PMC article. Review.
Cited by
-
Enteroendocrine peptides regulate feeding behavior via controlling intestinal contraction of the silkworm Bombyx mori.PLoS One. 2019 Jul 1;14(7):e0219050. doi: 10.1371/journal.pone.0219050. eCollection 2019. PLoS One. 2019. PMID: 31260470 Free PMC article.
-
Using machine learning to predict protein-protein interactions between a zombie ant fungus and its carpenter ant host.Sci Rep. 2023 Aug 24;13(1):13821. doi: 10.1038/s41598-023-40764-8. Sci Rep. 2023. PMID: 37620441 Free PMC article.
-
There is no magic bullet: the importance of testing reference gene stability in RT-qPCR experiments across multiple closely related species.PeerJ. 2020 Aug 3;8:e9618. doi: 10.7717/peerj.9618. eCollection 2020. PeerJ. 2020. PMID: 32832268 Free PMC article.
-
Endogenous l- to d-amino acid residue isomerization modulates selectivity between distinct neuropeptide receptor family members.Proc Natl Acad Sci U S A. 2023 Mar 14;120(11):e2217604120. doi: 10.1073/pnas.2217604120. Epub 2023 Mar 6. Proc Natl Acad Sci U S A. 2023. PMID: 36877849 Free PMC article.
References
-
- Badisco L., Huybrechts J., Simonet G., Verlinden H., Marchal E., Huybrechts R., et al. . (2011b). Transcriptome analysis of the desert locust central nervous system: production and annotation of a Schistocerca gregaria EST database. PLoS ONE 6:e17274. 10.1371/journal.pone.0017274 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

