The choroid plexus-a multi-role player during infectious diseases of the CNS
- PMID: 25814932
- PMCID: PMC4357259
- DOI: 10.3389/fncel.2015.00080
The choroid plexus-a multi-role player during infectious diseases of the CNS
Abstract
The choroid plexus (CP) is the source of cerebrospinal fluid (CSF) production and location of the blood-CSF barrier (BCSFB), which is constituted by the epithelial cells of the CP. Several infectious pathogens including viruses, bacteria, fungi and parasites cross the BCSFB to enter the central nervous system (CNS), ultimately leading to inflammatory infectious diseases like meningitis and meningoencephalitis. The CP responds to this challenge by the production of chemokines and cytokines as well as alterations of the barrier function of the BCSFB. During the course of CNS infectious disease host immune cells enter the CNS, eventually contributing to the cellular damage caused by the disease. Additional complications, which are in certain cases caused by choroid plexitis, can arise due to the response of the CP to the pathogens. In this review we will give an overview on the multiple functions of the CP during brain infections highlighting the CP as a multi-role player during infectious diseases of the CNS. In this context the importance of tools for investigation of these CP functions and a possible suitability of the CP as therapeutic target will be discussed.
Keywords: blood-brain barrier; blood-cerebrospinal fluid barrier; central nervous system infection and inflammation; choroid plexus; pathogen.
Figures
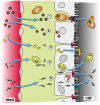
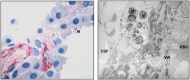
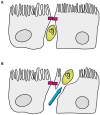
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

