Taurolithocholic acid promotes intrahepatic cholangiocarcinoma cell growth via muscarinic acetylcholine receptor and EGFR/ERK1/2 signaling pathway
- PMID: 25815516
- PMCID: PMC4441291
- DOI: 10.3892/ijo.2015.2939
Taurolithocholic acid promotes intrahepatic cholangiocarcinoma cell growth via muscarinic acetylcholine receptor and EGFR/ERK1/2 signaling pathway
Abstract
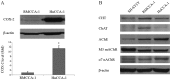
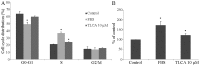
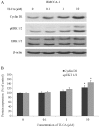
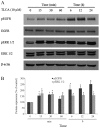
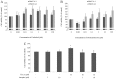
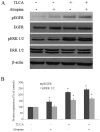
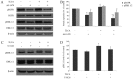
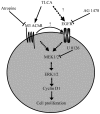
Cholangiocarcinoma (CCA) is a malignant cancer of the biliary tract and its occurrence is associated with chronic cholestasis which causes an elevation of bile acids in the liver and bile duct. The present study aimed to investigate the role and mechanistic effect of bile acids on the CCA cell growth. Intrahepatic CCA cell lines, RMCCA-1 and HuCCA-1, were treated with bile acids and their metabolites to determine the growth promoting effect. Cell viability, cell cycle analysis, EdU incorporation assays were conducted. Intracellular signaling proteins were detected by western immunoblotting. Among eleven forms of bile acids and their metabolites, only taurolithocholic acid (TLCA) concentration dependently (1-40 µM) increased the cell viability of RMCCA-1, but not HuCCA-1 cells. The cell cycle analysis showed induction of cells in the S phase and the EdU incorporation assay revealed induction of DNA synthesis in the TLCA-treated RMCCA-1 cells. Moreover, TLCA increased the phosphorylation of EGFR, ERK 1/2 and also increased the expression of cyclin D1 in RMCCA-1 cells. Furthermore, TLCA-induced RMCCA-1 cell growth could be inhibited by atropine, a non-selective muscarinic acetylcholine receptor (mAChR) antagonist, AG 1478, a specific EGFR inhibitor, or U 0126, a specific MEK 1/2 inhibitor. These results suggest that TLCA induces CCA cell growth via mAChR and EGFR/EKR1/2 signaling pathway. Moreover, the functional presence of cholinergic system plays a certain role in TLCA-induced CCA cell growth.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

