A low-cost wheat bran medium for biodegradation of the benzidine-based carcinogenic dye Trypan Blue using a microbial consortium
- PMID: 25815522
- PMCID: PMC4410198
- DOI: 10.3390/ijerph120403480
A low-cost wheat bran medium for biodegradation of the benzidine-based carcinogenic dye Trypan Blue using a microbial consortium
Abstract
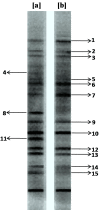
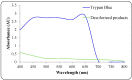
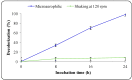
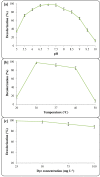
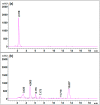
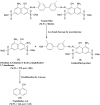
Environmental release of benzidine-based dyes is a matter of health concern. Here, a microbial consortium was enriched from textile dye contaminated soils and investigated for biodegradation of the carcinogenic benzidine-based dye Trypan Blue using wheat bran (WB) as growth medium. The PCR-DGGE analysis of enriched microbial consortium revealed the presence of 15 different bacteria. Decolorization studies suggested that the microbial consortium has high metabolic activity towards Trypan Blue as complete removal of 50 mg∙L-1 dye was observed within 24 h at 30 ± 0.2 °C and pH 7. Significant reduction in TOC (64%) and COD (88%) of dye decolorized broths confirmed mineralization. Induction in azoreductase (500%), NADH-DCIP reductase (264%) and laccase (275%) proved enzymatic decolorization of dye. HPLC analysis of dye decolorized products showed the formation of six metabolites while the FTIR spectrum indicated removal of diazo bonds at 1612.30 and 1581.34 cm-1. The proposed dye degradation pathway based on GC-MS and enzyme analysis suggested the formation of two low molecular weight intermediates. Phytotoxicity and acute toxicity studies revealed the less toxic nature of the dye degradation products. These results provide experimental evidence for the utilization of agricultural waste as a novel low-cost growth medium for biodegradation of benzidine-based dyes, and suggested the potential of the microbial consortium in detoxification.
Figures
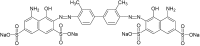

Similar articles
-
Mineralization and Detoxification of the Carcinogenic Azo Dye Congo Red and Real Textile Effluent by a Polyurethane Foam Immobilized Microbial Consortium in an Upflow Column Bioreactor.Int J Environ Res Public Health. 2015 Jun 16;12(6):6894-918. doi: 10.3390/ijerph120606894. Int J Environ Res Public Health. 2015. PMID: 26086710 Free PMC article.
-
Development of a bioreactor for remediation of textile effluent and dye mixture: a plant-bacterial synergistic strategy.Water Res. 2013 Mar 1;47(3):1035-48. doi: 10.1016/j.watres.2012.11.007. Epub 2012 Nov 23. Water Res. 2013. PMID: 23245543
-
Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR.Bioresour Technol. 2009 May;100(9):2493-500. doi: 10.1016/j.biortech.2008.12.013. Epub 2009 Jan 20. Bioresour Technol. 2009. PMID: 19157864
-
Phytoremediation of textile dyes and effluents: Current scenario and future prospects.Biotechnol Adv. 2015 Dec;33(8):1697-714. doi: 10.1016/j.biotechadv.2015.09.003. Epub 2015 Sep 18. Biotechnol Adv. 2015. PMID: 26386310 Review.
-
Detoxification of azo dyes in the context of environmental processes.Chemosphere. 2016 Jul;155:591-605. doi: 10.1016/j.chemosphere.2016.04.068. Epub 2016 May 4. Chemosphere. 2016. PMID: 27155475 Review.
Cited by
-
Chitosan-Grafted Halloysite Nanotubes-Fe3O4 Composite for Laccase-Immobilization and Sulfamethoxazole-Degradation.Polymers (Basel). 2020 Sep 27;12(10):2221. doi: 10.3390/polym12102221. Polymers (Basel). 2020. PMID: 32992644 Free PMC article.
-
Biodegradation of Azo Dye Methyl Red by Pseudomonas aeruginosa: Optimization of Process Conditions.Int J Environ Res Public Health. 2022 Aug 12;19(16):9962. doi: 10.3390/ijerph19169962. Int J Environ Res Public Health. 2022. PMID: 36011598 Free PMC article.
-
Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia.Saudi J Biol Sci. 2021 Jan;28(1):793-804. doi: 10.1016/j.sjbs.2020.11.012. Epub 2020 Nov 11. Saudi J Biol Sci. 2021. PMID: 33424369 Free PMC article.
-
Novel bacterial biofilm consortia that degrade and detoxify the carcinogenic diazo dye Congo red.Arch Microbiol. 2021 Mar;203(2):643-654. doi: 10.1007/s00203-020-02044-1. Epub 2020 Oct 6. Arch Microbiol. 2021. PMID: 33021681
-
Mineralization and Detoxification of the Carcinogenic Azo Dye Congo Red and Real Textile Effluent by a Polyurethane Foam Immobilized Microbial Consortium in an Upflow Column Bioreactor.Int J Environ Res Public Health. 2015 Jun 16;12(6):6894-918. doi: 10.3390/ijerph120606894. Int J Environ Res Public Health. 2015. PMID: 26086710 Free PMC article.
References
-
- US EPA Dyes Derived from Benzidine and Its Congeners. U.S. Environmental Protection Agency. [(accessed on 2 March 2015)];2010 Available online: http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/benzidine.html.
-
- CEPA (Canadian Environmental Protection Act., 1999): Notice with Respect to Certain Aromatic Amines and Aromatic Azo- and Benzidine-Based Substances. Canada Gazette, Part I, Volume 145, No. 51, Supplement to the Canada Gazette. [(accessed on 2 March 2015)]. Available online: www.gazette.gc.ca/rp-pr/p1/2011/index-eng.html.
-
- Morton K.C., King K.M., Baetcke K.P. Metabolism of benzidine and subsequent nucleic acid binding and mutagenicity. Cancer Res. 1979;39:3107–3113. - PubMed
-
- Combes R.D., Haveland-Smith R.B. A review of the genotoxicity of food, drug and cosmetic colours and other azo triphenylmethane and xanthene dyes. Mutat. Res. 1982;98:101–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

