Post-transcriptional regulation of satellite cell quiescence by TTP-mediated mRNA decay
- PMID: 25815583
- PMCID: PMC4415119
- DOI: 10.7554/eLife.03390
Post-transcriptional regulation of satellite cell quiescence by TTP-mediated mRNA decay
Abstract
Skeletal muscle satellite cells in their niche are quiescent and upon muscle injury, exit quiescence, proliferate to repair muscle tissue, and self-renew to replenish the satellite cell population. To understand the mechanisms involved in maintaining satellite cell quiescence, we identified gene transcripts that were differentially expressed during satellite cell activation following muscle injury. Transcripts encoding RNA binding proteins were among the most significantly changed and included the mRNA decay factor Tristetraprolin. Tristetraprolin promotes the decay of MyoD mRNA, which encodes a transcriptional regulator of myogenic commitment, via binding to the MyoD mRNA 3' untranslated region. Upon satellite cell activation, p38α/β MAPK phosphorylates MAPKAP2 and inactivates Tristetraprolin, stabilizing MyoD mRNA. Satellite cell specific knockdown of Tristetraprolin precociously activates satellite cells in vivo, enabling MyoD accumulation, differentiation and cell fusion into myofibers. Regulation of mRNAs by Tristetraprolin appears to function as one of several critical post-transcriptional regulatory mechanisms controlling satellite cell homeostasis.
Keywords: developmental biology; homeostasis; mouse; niche; quiescence; regeneration; skeletal muscle; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




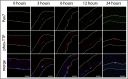
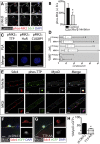







References
-
- Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR. Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Molecular and Cellular Biology. 2006;26:2408–2418. doi: 10.1128/MCB.26.6.2408-2418.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

