Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis
- PMID: 25815810
- PMCID: PMC4376793
- DOI: 10.1371/journal.pgen.1005120
Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis
Abstract
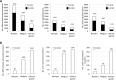
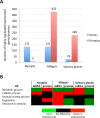
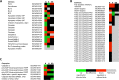
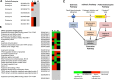
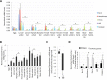
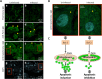
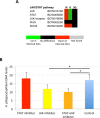
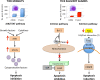
Anaplasma phagocytophilum is an emerging pathogen that causes human granulocytic anaplasmosis. Infection with this zoonotic pathogen affects cell function in both vertebrate host and the tick vector, Ixodes scapularis. Global tissue-specific response and apoptosis signaling pathways were characterized in I. scapularis nymphs and adult female midguts and salivary glands infected with A. phagocytophilum using a systems biology approach combining transcriptomics and proteomics. Apoptosis was selected for pathway-focused analysis due to its role in bacterial infection of tick cells. The results showed tissue-specific differences in tick response to infection and revealed differentiated regulation of apoptosis pathways. The impact of bacterial infection was more pronounced in tick nymphs and midguts than in salivary glands, probably reflecting bacterial developmental cycle. All apoptosis pathways described in other organisms were identified in I. scapularis, except for the absence of the Perforin ortholog. Functional characterization using RNA interference showed that Porin knockdown significantly increases tick colonization by A. phagocytophilum. Infection with A. phagocytophilum produced complex tissue-specific alterations in transcript and protein levels. In tick nymphs, the results suggested a possible effect of bacterial infection on the inhibition of tick immune response. In tick midguts, the results suggested that A. phagocytophilum infection inhibited cell apoptosis to facilitate and establish infection through up-regulation of the JAK/STAT pathway. Bacterial infection inhibited the intrinsic apoptosis pathway in tick salivary glands by down-regulating Porin expression that resulted in the inhibition of Cytochrome c release as the anti-apoptotic mechanism to facilitate bacterial infection. However, tick salivary glands may promote apoptosis to limit bacterial infection through induction of the extrinsic apoptosis pathway. These dynamic changes in response to A. phagocytophilum in I. scapularis tissue-specific transcriptome and proteome demonstrated the complexity of the tick response to infection and will contribute to characterize gene regulation in ticks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- de la Fuente J, Estrada-Peña A, Venzal JM, Kocan KM, Sonenshine DE (2008) Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci 13: 6938–6946. - PubMed
-
- Reichard MV, Manzano-Roman R, Kocan KM, Blouin EF, de la Fuente J, et al. (2009) Inoculation of white-tailed deer (Odocoileus virginianus) with Ap-V1 or NY-18 strains of Anaplasma phagocytophilum and microscopic demonstration of Ap-V1 in Ixodes scapularis adults that acquired infection from deer as nymphs. Vector Borne Zoonotic Dis 9: 565–568. 10.1089/vbz.2008.0106 - DOI - PubMed
-
- Dumler JS, Barbet AC, Bekker CPJ, Dasch GA, Palmer GH, et al. (2001) Reorganization of the genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions subjective synonyms of Ehrlichia phagocytophila . Int J Syst Evol Microbiol 51: 2145–2165. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

