Immune regulation of metabolic homeostasis in health and disease
- PMID: 25815992
- PMCID: PMC4400287
- DOI: 10.1016/j.cell.2015.02.022
Immune regulation of metabolic homeostasis in health and disease
Abstract
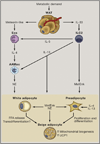
Obesity is an increasingly prevalent disease worldwide. While genetic and environmental factors are known to regulate the development of obesity and associated metabolic diseases, emerging studies indicate that innate and adaptive immune cell responses in adipose tissue have critical roles in the regulation of metabolic homeostasis. In the lean state, type 2 cytokine-associated immune cell responses predominate in white adipose tissue and protect against weight gain and insulin resistance through direct effects on adipocytes and elicitation of beige adipose. In obesity, these metabolically beneficial immune pathways become dysregulated, and adipocytes and other factors initiate metabolically deleterious type 1 inflammation that impairs glucose metabolism. This review discusses our current understanding of the functions of different types of adipose tissue and how immune cells regulate adipocyte function and metabolic homeostasis in the context of health and disease and highlights. We also highlight the potential of targeting immuno-metabolic pathways as a therapeutic strategy to treat obesity and associated diseases.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO molecular medicine. 2011;3:559–572. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- T32-AI060516/AI/NIAID NIH HHS/United States
- F30-AI112023/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- AI061570/AI/NIAID NIH HHS/United States
- P01 AI106697/AI/NIAID NIH HHS/United States
- AI102942/AI/NIAID NIH HHS/United States
- F30 AI112023/AI/NIAID NIH HHS/United States
- AI106697/AI/NIAID NIH HHS/United States
- AI074878/AI/NIAID NIH HHS/United States
- T32 AI060516/AI/NIAID NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- AI095608/AI/NIAID NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- R01 AI097333/AI/NIAID NIH HHS/United States
- AI095466/AI/NIAID NIH HHS/United States
- AI097333/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

