A GFP expressing influenza A virus to report in vivo tropism and protection by a matrix protein 2 ectodomain-specific monoclonal antibody
- PMID: 25816132
- PMCID: PMC4376807
- DOI: 10.1371/journal.pone.0121491
A GFP expressing influenza A virus to report in vivo tropism and protection by a matrix protein 2 ectodomain-specific monoclonal antibody
Abstract
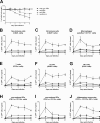
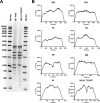
The severity of influenza-related illness is mediated by many factors, including in vivo cell tropism, timing and magnitude of the immune response, and presence of pre-existing immunity. A direct way to study cell tropism and virus spread in vivo is with an influenza virus expressing a reporter gene. However, reporter gene-expressing influenza viruses are often attenuated in vivo and may be genetically unstable. Here, we describe the generation of an influenza A virus expressing GFP from a tri-cistronic NS segment. To reduce the size of this engineered gene segment, we used a truncated NS1 protein of 73 amino acids combined with a heterologous dimerization domain to increase protein stability. GFP and nuclear export protein coding information were fused in frame with the truncated NS1 open reading frame and separated from each other by 2A self-processing sites. The resulting PR8-NS1(1-73)GFP virus was successfully rescued and replicated as efficiently as the parental PR8 virus in vitro and was slightly attenuated in vivo. Flow cytometry-based monitoring of cells isolated from PR8-NS1(1-73)GFP virus infected BALB/c mice revealed that GFP expression peaked on day two in all cell types tested. In particular respiratory epithelial cells and myeloid cells known to be involved in antigen presentation, including dendritic cells (CD11c+) and inflammatory monocytes (CD11b+ GR1+), became GFP positive following infection. Prophylactic treatment with anti-M2e monoclonal antibody or oseltamivir reduced GFP expression in all cell types studied, demonstrating the usefulness of this reporter virus to analyze the efficacy of antiviral treatments in vivo. Finally, deep sequencing analysis, serial in vitro passages and ex vivo analysis of PR8-NS1(1-73)GFP virus, indicate that this virus is genetically and phenotypically stable.
Conflict of interest statement
Figures


References
-
- Katz JM, Plowden J, Renshaw-Hoelscher M, Lu X, Tumpey TM, Sambhara S. Immunity to influenza: the challenges of protecting an aging population. Immunologic research. 2004;29(1–3):113–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

