Pulmonary inflammation is regulated by the levels of the vesicular acetylcholine transporter
- PMID: 25816137
- PMCID: PMC4376856
- DOI: 10.1371/journal.pone.0120441
Pulmonary inflammation is regulated by the levels of the vesicular acetylcholine transporter
Abstract
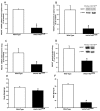
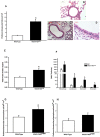
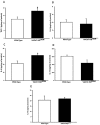
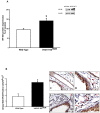
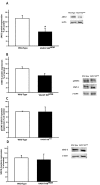
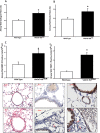
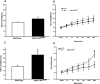
Acetylcholine (ACh) plays a crucial role in physiological responses of both the central and the peripheral nervous system. Moreover, ACh was described as an anti-inflammatory mediator involved in the suppression of exacerbated innate response and cytokine release in various organs. However, the specific contributions of endogenous release ACh for inflammatory responses in the lung are not well understood. To address this question we have used mice with reduced levels of the vesicular acetylcholine transporter (VAChT), a protein required for ACh storage in secretory vesicles. VAChT deficiency induced airway inflammation with enhanced TNF-α and IL-4 content, but not IL-6, IL-13 and IL-10 quantified by ELISA. Mice with decreased levels of VAChT presented increased collagen and elastic fibers deposition in airway walls which was consistent with an increase in inflammatory cells positive to MMP-9 and TIMP-1 in the lung. In vivo lung function evaluation showed airway hyperresponsiveness to methacholine in mutant mice. The expression of nuclear factor-kappa B (p65-NF-kB) in lung of VAChT-deficient mice were higher than in wild-type mice, whereas a decreased expression of janus-kinase 2 (JAK2) was observed in the lung of mutant animals. Our findings show the first evidence that cholinergic deficiency impaired lung function and produce local inflammation. Our data supports the notion that cholinergic system modulates airway inflammation by modulation of JAK2 and NF-kB pathway. We proposed that intact cholinergic pathway is necessary to maintain the lung homeostasis.
Conflict of interest statement
Figures

References
-
- Postma DS, Timens W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3: 434–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

