Kinetically-defined component actions in gene repression
- PMID: 25816223
- PMCID: PMC4376387
- DOI: 10.1371/journal.pcbi.1004122
Kinetically-defined component actions in gene repression
Abstract
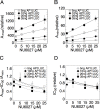
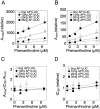
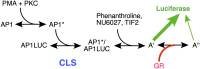
Gene repression by transcription factors, and glucocorticoid receptors (GR) in particular, is a critical, but poorly understood, physiological response. Among the many unresolved questions is the difference between GR regulated induction and repression, and whether transcription cofactor action is the same in both. Because activity classifications based on changes in gene product level are mechanistically uninformative, we present a theory for gene repression in which the mechanisms of factor action are defined kinetically and are consistent for both gene repression and induction. The theory is generally applicable and amenable to predictions if the dose-response curve for gene repression is non-cooperative with a unit Hill coefficient, which is observed for GR-regulated repression of AP1LUC reporter induction by phorbol myristate acetate. The theory predicts the mechanism of GR and cofactors, and where they act with respect to each other, based on how each cofactor alters the plots of various kinetic parameters vs. cofactor. We show that the kinetically-defined mechanism of action of each of four factors (reporter gene, p160 coactivator TIF2, and two pharmaceuticals [NU6027 and phenanthroline]) is the same in GR-regulated repression and induction. What differs is the position of GR action. This insight should simplify clinical efforts to differentially modulate factor actions in gene induction vs. gene repression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Pratt WB, Toft DO (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18: 306–360. - PubMed
-
- Lonard DM, O’Malley BW (2007) Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27: 691–700. - PubMed
-
- Pascual-Le Tallec L, Simone F, Viengchareun S, Meduri G, Thirman MJ et al. (2005) The elongation factor ELL (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions. Mol Endocrinol 19: 1158–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

