Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy
- PMID: 25816249
- PMCID: PMC4376686
- DOI: 10.1371/journal.pone.0122151
Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy
Abstract
Purpose: To evaluate diffusion weighted MRI (DW-MR) as a response metric for assessment of neoadjuvant chemotherapy (NAC) in patients with primary breast cancer using prospective multi-center trials which provided MR scans along with clinical outcome information.
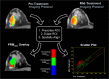
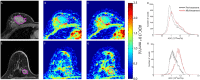
Materials and methods: A total of 39 patients with locally advanced breast cancer accrued from three different prospective clinical trials underwent DW-MR examination prior to and at 3-7 days (Hull University), 8-11 days (University of Michigan) and 35 days (NeoCOMICE) post-treatment initiation. Thirteen patients, 12 of which participated in treatment response study, from UM underwent short interval (<1hr) MRI examinations, referred to as "test-retest" for examination of repeatability. To further evaluate stability in ADC measurements, a thermally controlled diffusion phantom was used to assess repeatability of diffusion measurements. MRI sequences included contrast-enhanced T1-weighted, when appropriate, and DW images acquired at b-values of 0 and 800 s/mm2. Histogram analysis and a voxel-based analytical technique, the Parametric Response Map (PRM), were used to derive diffusion response metrics for assessment of treatment response prediction.
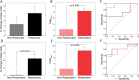
Results: Mean tumor apparent diffusion coefficient (ADC) values generated from patient test-retest examinations were found to be very reproducible (|ΔADC|<0.1x10-3mm2/s). This data was used to calculate the 95% CI from the linear fit of tumor voxel ADC pairs of co-registered examinations (±0.45x10-3mm2/s) for PRM analysis of treatment response. Receiver operating characteristic analysis identified the PRM metric to be predictive of outcome at the 8-11 (AUC = 0.964, p = 0.01) and 35 day (AUC = 0.770, p = 0.05) time points (p<.05) while whole-tumor ADC changes where significant at the later 35 day time interval (AUC = 0.825, p = 0.02).
Conclusion: This study demonstrates the feasibility of performing a prospective analysis of DW-MRI as a predictive biomarker of NAC in breast cancer patients. In addition, we provide experimental evidence supporting the use of sensitive analytical tools, such as PRM, for evaluating ADC measurements.
Conflict of interest statement
Figures



References
-
- Baum M. Polychemotherapy for early breast cancer. Lancet. 1998; 352: 1554 - PubMed
-
- Saibara T, Ogawa Y, Onishi S. Tamoxifen in early breast cancer. Lancet. 1998; 352: 404 - PubMed
-
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005; 23: 3676–3685. - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005; 353: 1673–1684. - PubMed
-
- Valero V, Buzdar AU, McNeese M, Singletary E, Hortobagyi GN. Primary chemotherapy in the treatment of breast cancer: the University of Texas M. D. Anderson Cancer Center experience. Clin Breast Cancer. 2002; 3 Suppl 2: 63–68. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

