Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin
- PMID: 25816775
- PMCID: PMC4392646
- DOI: 10.1016/j.chom.2015.03.001
Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin
Abstract
Resistance and tolerance are two defense strategies employed by the host against microbial threats. Autophagy-mediated degradation of bacteria has been extensively described as a major resistance mechanism. Here we find that the dominant function of autophagy proteins during infections with the epidemic community-associated methicillin-resistant Staphylococcus aureus USA300 is to mediate tolerance rather than resistance. Atg16L1 hypomorphic mice (Atg16L1(HM)), which have reduced autophagy, were highly susceptible to lethality in both sepsis and pneumonia models of USA300 infection. Autophagy confers protection by limiting the damage caused by α-toxin, particularly to endothelial cells. Remarkably, Atg16L1(HM) mice display enhanced survival rather than susceptibility upon infection with α-toxin-deficient S. aureus. These results identify an essential role for autophagy in tolerance to Staphylococcal disease and highlight how a single virulence factor encoded by a pathogen can determine whether a given host factor promotes tolerance or resistance.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



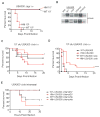
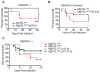
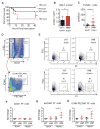
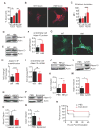
Comment in
-
Host autophagy combating S. aureus: α-toxin will be tolerated.Cell Host Microbe. 2015 Apr 8;17(4):419-20. doi: 10.1016/j.chom.2015.03.010. Cell Host Microbe. 2015. PMID: 25856749 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- F30 DK098925/DK/NIDDK NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- R01 DK093668/DK/NIDDK NIH HHS/United States
- R01AI099394/AI/NIAID NIH HHS/United States
- R01AI105129/AI/NIAID NIH HHS/United States
- AI100853/AI/NIAID NIH HHS/United States
- R01 AI099394/AI/NIAID NIH HHS/United States
- 5 T32GM0738/GM/NIGMS NIH HHS/United States
- 5P30CA016087-33/CA/NCI NIH HHS/United States
- P30CA16087/CA/NCI NIH HHS/United States
- R01 AI105129/AI/NIAID NIH HHS/United States
- F31 AI112290/AI/NIAID NIH HHS/United States
- RR023704-01A1/RR/NCRR NIH HHS/United States
- T32 AI007180/AI/NIAID NIH HHS/United States
- T32 AI100853/AI/NIAID NIH HHS/United States
- S10 RR023704/RR/NCRR NIH HHS/United States
- F32 AI098395/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

