Visual contribution to human standing balance during support surface tilts
- PMID: 25816794
- PMCID: PMC4427279
- DOI: 10.1016/j.humov.2015.02.010
Visual contribution to human standing balance during support surface tilts
Abstract
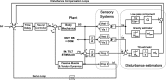
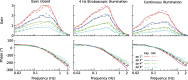
Visual position and velocity cues improve human standing balance, reducing sway responses to external disturbances and sway variability. Previous work suggested that human balancing is based on sensory estimates of external disturbances and their compensation using feedback mechanisms (Disturbance Estimation and Compensation, DEC model). This study investigates the visual effects on sway responses to pseudo-random support surface tilts, assuming that improvements result from lowering the velocity threshold in a tilt estimate and the position threshold in an estimate of the gravity disturbance. Center of mass (COM) sway was measured with four different tilt amplitudes, separating the effect of visual cues across the conditions 'Eyes closed' (no visual cues), '4Hz stroboscopic illumination' (visual position cues), and 'continuous illumination' (visual position and velocity cues). In a model based approach, parameters of disturbance estimators were identified. The model reproduced experimental results and showed a specific reduction of the position and velocity threshold when adding visual position and velocity cues, respectively. Sway variability was analyzed to explore a hypothesized relation between estimator thresholds and internal noise. Results suggest that adding the visual cues reduces the contribution of vestibular noise, thereby reducing sway variability and allowing for lower thresholds, which improves the disturbance compensation.
Keywords: Human posture control; Model simulations; Noise; Sensory and Motor Testing; Stroboscopic illumination; Visual cues.
Copyright © 2015 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Amblard B., Crémieux J., Marchand A.R., Carblanc A. Lateral orientation and stabilization of human stance: static versus dynamic visual cues. Experimental Brain Research. 1985;61(1):21–37. - PubMed
-
- Assländer L., Hettich G., Gollhofer A., Mergner T. Contribution of visual velocity and displacement cues to human balancing of support surface tilt. Experimental Brain Research. 2013;228(3):297–304. - PubMed
-
- Bach M., Kommerell G. Determining visual acuity using European normal values: scientific principles and possibilities for automatic measurement. Klinische Monatsblätter Für Augenheilkunde. 1998;212(4):190–195. - PubMed
-
- Blümle A., Maurer C., Schweigart G., Mergner T. A cognitive intersensory interaction mechanism in human postural control. Experimental Brain Research. 2006;173(3):357–363. - PubMed
-
- Bosco G., Poppele R.E. Representation of multiple kinematic parameters of the cat hindlimb in spinocerebellar activity. Journal of Neurophysiology. 1997;78(3):1421–1432. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

