Pharmacological inhibition of PI3K reduces adiposity and metabolic syndrome in obese mice and rhesus monkeys
- PMID: 25817535
- PMCID: PMC5867518
- DOI: 10.1016/j.cmet.2015.02.017
Pharmacological inhibition of PI3K reduces adiposity and metabolic syndrome in obese mice and rhesus monkeys
Abstract
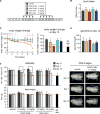
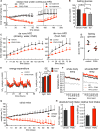
Genetic inhibition of PI3K signaling increases energy expenditure, protects from obesity and metabolic syndrome, and extends longevity. Here, we show that two pharmacological inhibitors of PI3K, CNIO-PI3Ki and GDC-0941, decrease the adiposity of obese mice without affecting their lean mass. Long-term treatment of obese mice with low doses of CNIO-PI3Ki reduces body weight until reaching a balance that is stable for months as long as the treatment continues. CNIO-PI3Ki treatment also ameliorates liver steatosis and decreases glucose serum levels. The above observations have been recapitulated in independent laboratories and using different oral formulations of CNIO-PI3Ki. Finally, daily oral treatment of obese rhesus monkeys for 3 months with low doses of CNIO-PI3Ki decreased their adiposity and lowered their serum glucose levels, in the absence of detectable toxicities. Therefore, pharmacological inhibition of PI3K is an effective and safe anti-obesity intervention that could reverse the negative effects of metabolic syndrome in humans.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Obesity: Inhibiting PI3K reduces body weight in obese mice.Nat Rev Endocrinol. 2015 Jun;11(6):317. doi: 10.1038/nrendo.2015.60. Epub 2015 Apr 7. Nat Rev Endocrinol. 2015. PMID: 25850662 No abstract available.
References
-
- Becattini B, Marone R, Zani F, Arsenijevic D, Seydoux J, Montani JP, Dulloo AG, Thorens B, Preitner F, Wymann MP, Solinas G. PI3Kγ within a nonhematopoietic cell type negatively regulates diet-induced thermogenesis and promotes obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:E854–E863. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

