Characterizing human vestibular sensory epithelia for experimental studies: new hair bundles on old tissue and implications for therapeutic interventions in ageing
- PMID: 25818177
- PMCID: PMC4436436
- DOI: 10.1016/j.neurobiolaging.2015.02.013
Characterizing human vestibular sensory epithelia for experimental studies: new hair bundles on old tissue and implications for therapeutic interventions in ageing
Abstract
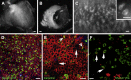
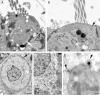
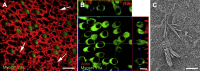
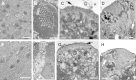
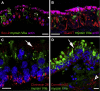
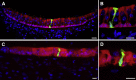
Balance disequilibrium is a significant contributor to falls in the elderly. The most common cause of balance dysfunction is loss of sensory cells from the vestibular sensory epithelia of the inner ear. However, inaccessibility of inner ear tissue in humans severely restricts possibilities for experimental manipulation to develop therapies to ameliorate this loss. We provide a structural and functional analysis of human vestibular sensory epithelia harvested at trans-labyrinthine surgery. We demonstrate the viability of the tissue and labeling with specific markers of hair cell function and of ion homeostasis in the epithelium. Samples obtained from the oldest patients revealed a significant loss of hair cells across the tissue surface, but we found immature hair bundles present in epithelia harvested from patients >60 years of age. These results suggest that the environment of the human vestibular sensory epithelium could be responsive to stimulation of developmental pathways to enhance hair cell regeneration, as has been demonstrated successfully in the vestibular organs of adult mice.
Keywords: Aging pathologies; Hair cells; Human vestibular; Stereocilia; Supporting cells; Utricle.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Adler H.J., Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci. Lett. 1996;205:17–20. - PubMed
-
- Bagger-Sjoback D., Lundquist P.G., Galey F., Ylikoski J. The sensory epithelia of the human labyrinth. A freeze-fracturing and transmission electron microscopic study. Am. J. Otol. 1983;4:203–213. - PubMed
-
- Baloh R.W., Enrietto J., Jacobson K.M., Lin A. Age-related changes in vestibular function: a longitudinal study. Ann. N. Y. Acad. Sci. 2001;942:210–219. - PubMed
-
- Bermingham N.A., Hassan B.A., Price S.D., Vollrath M.A., Ben-Arie N., Eatock R.A., Bellen H.J., Lysakowski A., Zoghbi H.Y. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

