Hypercoagulability in dogs with blastomycosis
- PMID: 25818206
- PMCID: PMC4895520
- DOI: 10.1111/jvim.12538
Hypercoagulability in dogs with blastomycosis
Abstract
Background: Blastomycosis is a potentially fatal fungal disease that most commonly affects humans and dogs. The organism causes systemic inflammation and has a predilection for the lungs. The inflammation might lead to a hypercoagulable state with microemboli in the pulmonary circulation which could contribute to inadequate oxygen exchange in infected dogs.
Hypothesis/objectives: Dogs with blastomycosis will be hypercoagulable compared with healthy case-matched controls.
Animals: Client-owned dogs with a diagnosis of blastomycosis (n = 23) and healthy case-matched controls (n = 23).
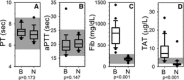
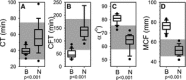
Methods: Prospective case-controlled study of client-owned dogs presented to a veterinary teaching hospital with clinical signs compatible with blastomycosis. Complete blood counts, fibrinogen, PT, aPTT, thromboelastometry (TE), thrombin antithrombin complexes (TAT), and thrombin generation were evaluated.
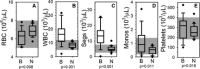
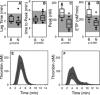
Results: Cases had a leukocytosis compared with controls [mean (SD) 16.6 (7.6) × 10(3)/μL versus 8.2 (1.8) × 10(3)/μL, P < .001], hyperfibrinogenemia [median 784 mg/dL, range 329-1,443 versus median 178 mg/dL, range 82-257, P < .001], and increased TAT concentrations [mean (SD) 9.0 (5.7) μg/L versus 2.0 (2.8) μg/L, P < .001]. As compared to controls, cases were also hypercoagulable as evaluated by thromboelastometry and had increased in vitro thrombin generation on calibrated automated thrombography.
Conclusions and clinical importance: Hypercoagulability occurs in dogs with systemic blastomycosis. Additional studies are needed to explore a possible contribution of thrombogenicity to the clinical manifestations of systemic blastomycosis.
Keywords: Canine; Thromboelastography; Thromboelastometry; Thrombosis.
Copyright © 2015 by the American College of Veterinary Internal Medicine.
Figures
Similar articles
-
Hemostatic abnormalities in dogs with carcinoma: a thromboelastographic characterization of hypercoagulability.Vet J. 2011 Nov;190(2):e78-e83. doi: 10.1016/j.tvjl.2011.02.025. Epub 2011 Mar 31. Vet J. 2011. PMID: 21454111
-
In vitro hypercoagulability on whole blood thromboelastometry associated with in vivo reduction of circulating red cell mass in dogs.Vet Clin Pathol. 2014 Jun;43(2):154-63. doi: 10.1111/vcp.12127. Epub 2014 Mar 3. Vet Clin Pathol. 2014. PMID: 24588681
-
Hemostatic profiles in dogs with sudden acquired retinal degeneration syndrome.J Vet Intern Med. 2023 May-Jun;37(3):948-959. doi: 10.1111/jvim.16710. Epub 2023 Apr 19. J Vet Intern Med. 2023. PMID: 37073895 Free PMC article.
-
Hypercoagulability in dogs: pathophysiology.Compend Contin Educ Vet. 2012 Apr;34(4):E1-5. Compend Contin Educ Vet. 2012. PMID: 22488600 Review.
-
Epidemiology, diagnosis, and treatment of blastomycosis in dogs and cats.Clin Tech Small Anim Pract. 2005 Nov;20(4):233-9. doi: 10.1053/j.ctsap.2005.07.004. Clin Tech Small Anim Pract. 2005. PMID: 16317913 Review.
Cited by
-
A Remote Assay for Measuring Canine Platelet Activation and the Inhibitory Effects of Antiplatelet Agents.J Vet Intern Med. 2018 Jan;32(1):119-127. doi: 10.1111/jvim.14845. Epub 2017 Dec 2. J Vet Intern Med. 2018. PMID: 29197128 Free PMC article.
-
Disseminated aspergillosis associated with severe non-regenerative anemia in a Lagotto Romagnolo: a case report.Vet Res Commun. 2025 Jul 2;49(4):243. doi: 10.1007/s11259-025-10809-6. Vet Res Commun. 2025. PMID: 40601090 No abstract available.
-
Haemostatic, fibrinolytic and inflammatory profiles in West Highland white terriers with canine idiopathic pulmonary fibrosis and controls.BMC Vet Res. 2019 Oct 29;15(1):379. doi: 10.1186/s12917-019-2134-z. BMC Vet Res. 2019. PMID: 31664993 Free PMC article.
-
A pilot study evaluating the Calibrated Automated Thrombogram assay and application of plasma-thromboelastography for detection of hemostatic aberrations in horses with gastrointestinal disease.BMC Vet Res. 2021 Nov 8;17(1):346. doi: 10.1186/s12917-021-03058-7. BMC Vet Res. 2021. PMID: 34749707 Free PMC article.
-
Correlation between thromboelastography and traditional coagulation test parameters in hospitalized dogs.Vet Med (Auckl). 2017 Feb 8;8:21-26. doi: 10.2147/VMRR.S122437. eCollection 2017. Vet Med (Auckl). 2017. PMID: 30050851 Free PMC article.
References
-
- Lemos LB, Baliga M, Guo M. Acute respiratory distress syndrome and blastomycosis: Presentation of nine cases and review of the literature. Ann Diagn Pathol 2001;5:1–9. - PubMed
-
- Vasquez JE, Mehta JB, Agrawal R, et al. Blastomycosis in northeast Tennessee. Chest 1998;114:436–443. - PubMed
-
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008;46:1801–1812. - PubMed
-
- Meyer KC, McManus EJ, Maki DG. Overwhelming pulmonary blastomycosis associated with the adult respiratory distress syndrome. N Engl J Med 1993;329:1231–1236. - PubMed
-
- Gauthier GM, Safdar N, Klein BS, et al. Blastomycosis in solid organ transplant recipients. Transpl Infect Dis 2007;9:310–317. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

