Pharmacokinetics of single-dose rectal zonisamide administration in normal dogs
- PMID: 25818215
- PMCID: PMC4895495
- DOI: 10.1111/jvim.12540
Pharmacokinetics of single-dose rectal zonisamide administration in normal dogs
Abstract
Background: Few medications are available for parental administration to animals with seizures. Rectal administration of medications is often used if the animal cannot be administered oral medications.
Hypothesis/objectives: To determine the pharmacokinetic differences in zonisamide when administered rectally in either of 2 vehicles and p.o. to dogs.
Animals: Eight healthy research dogs.
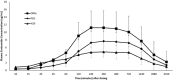
Methods: Randomized cross-over design. Zonisamide, 10 mg/kg, was administered rectally in polyethylene glycol (PEG-R), rectally in water (H2O-R), and as an oral capsule. Plasma zonisamide concentrations were measured until 72 hours after administration. Zonisamide was quantitated by HPLC and plasma concentration versus time curve data was analyzed by using noncompartmental modeling.
Results: Mean maximum plasma zonisamide concentrations (μg/mL) were significantly higher after oral administration (11.56 ± 4.04) compared to H2O-R (5.00 ± 1.83) (P = .004). Disappearance half-life (hours) and mean time to maximum concentration (hours) were not significantly different between methods of administration. Mean relative bioavailability of PEG-R (85 ± 69%) was significantly higher than that of H2O-R (53 ± 37%) (P = .039). Dogs tolerated all dosing forms with no evidence of adverse effects.
Conclusions and clinical importance: The vehicle in which zonisamide is dissolved influences rectal bioavailability, with PEG preferred to H2O-R. Because of the prolonged time to maximum concentration, rectal administration of zonisamide should not be used to treat status epilepticus in dogs. A dose higher than what was used in this study might be necessary, if currently recommended minimum therapeutic concentrations (10 μg/mL) are to be achieved with a single-dose administration.
Keywords: Bioavailability; Epilepsy; Polyethylene glycol; Seizure; Status epilepticus.
Copyright © 2014 by the American College of Veterinary Internal Medicine.
Figures
Similar articles
-
Pharmacokinetics of zonisamide following rectal administration to healthy dogs.Am J Vet Res. 2016 Dec;77(12):1374-1380. doi: 10.2460/ajvr.77.12.1374. Am J Vet Res. 2016. PMID: 27901384 Clinical Trial.
-
Disposition and safety of zonisamide after intravenous and oral single dose and oral multiple dosing in normal hound dogs.J Vet Pharmacol Ther. 2008 Dec;31(6):544-53. doi: 10.1111/j.1365-2885.2008.00993.x. J Vet Pharmacol Ther. 2008. PMID: 19000278
-
Pharmacokinetics of zonisamide and drug interaction with phenobarbital in dogs.J Vet Pharmacol Ther. 2008 Jun;31(3):259-64. doi: 10.1111/j.1365-2885.2008.00955.x. J Vet Pharmacol Ther. 2008. PMID: 18471148
-
Profile of once-daily zonisamide as monotherapy for treatment of partial seizures in adults.Drug Des Devel Ther. 2013 May 14;7:397-402. doi: 10.2147/DDDT.S43612. Print 2013. Drug Des Devel Ther. 2013. PMID: 23700365 Free PMC article. Review.
-
Zonisamide for the treatment of Parkinson's disease.Expert Rev Neurother. 2007 Sep;7(9):1077-83. doi: 10.1586/14737175.7.9.1077. Expert Rev Neurother. 2007. PMID: 17868006 Review.
Cited by
-
ACVIM Consensus Statement on the management of status epilepticus and cluster seizures in dogs and cats.J Vet Intern Med. 2024 Jan-Feb;38(1):19-40. doi: 10.1111/jvim.16928. Epub 2023 Nov 3. J Vet Intern Med. 2024. PMID: 37921621 Free PMC article.
-
Comparative single-dose pharmacokinetics of sildenafil after oral and rectal administration in healthy beagle dogs.BMC Vet Res. 2018 Sep 24;14(1):291. doi: 10.1186/s12917-018-1617-7. BMC Vet Res. 2018. PMID: 30249242 Free PMC article.
-
Pharmacokinetics of Pimobendan and Its Metabolite O-Desmethyl-Pimobendan Following Rectal Administration to Healthy Dogs.Front Vet Sci. 2020 Aug 4;7:423. doi: 10.3389/fvets.2020.00423. eCollection 2020. Front Vet Sci. 2020. PMID: 32851013 Free PMC article.
-
Immune-mediated polyarthritis and anterior uveitis secondary to zonisamide administration in a dog with refractory epilepsy.Vet Med Sci. 2024 Mar;10(2):e1374. doi: 10.1002/vms3.1374. Vet Med Sci. 2024. PMID: 38403976 Free PMC article.
References
-
- Thomas WB, Dewey CW. Seizures and narcolepsy In: Dewey CW, ed. A Practical Guide to Canine and Feline Neurology, 2nd ed Ames, IA: Wiley‐Blackwell; 2008:237.
-
- Jaggy A, Bernardini M. Idiopathic epilepsy in 125 dogs: A long‐term study. Clinical and electroencephalographic findings. J Small Anim Pract 1998;39:23–29. - PubMed
-
- Mariani C. Terminology and classification of seizures and epilepsy in veterinary patients. Top Companion Anim Med 2013;28:34–41. - PubMed
-
- Dewey CW. Anticonvulsant therapy in dogs and cats. Vet Clin North Am Small Anim Pract 2006;36:1107–1127, vii. - PubMed
-
- Podell M, Fenner WR. Bromide therapy in refractory canine idiopathic epilepsy. J Vet Intern Med 1993;7:318–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources