3D surface topology guides stem cell adhesion and differentiation
- PMID: 25818420
- PMCID: PMC4379418
- DOI: 10.1016/j.biomaterials.2015.01.034
3D surface topology guides stem cell adhesion and differentiation
Abstract
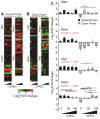
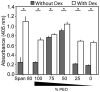
Polymerized high internal phase emulsion (polyHIPE) foams are extremely versatile materials for investigating cell-substrate interactions in vitro. Foam morphologies can be controlled by polymerization conditions to result in either open or closed pore structures with different levels of connectivity, consequently enabling the comparison between 2D and 3D matrices using the same substrate with identical surface chemistry conditions. Additionally, here we achieve the control of pore surface topology (i.e. how different ligands are clustered together) using amphiphilic block copolymers as emulsion stabilizers. We demonstrate that adhesion of human mesenchymal progenitor (hES-MP) cells cultured on polyHIPE foams is dependent on foam surface topology and chemistry but is independent of porosity and interconnectivity. We also demonstrate that the interconnectivity, architecture and surface topology of the foams has an effect on the osteogenic differentiation potential of hES-MP cells. Together these data demonstrate that the adhesive heterogeneity of a 3D scaffold could regulate not only mesenchymal stem cell attachment but also cell behavior in the absence of soluble growth factors.
Keywords: Cell signaling; Osteogenesis; Stem cell; Surface topology.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. - PubMed
-
- Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–8. - PubMed
-
- Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55–62. - PubMed
-
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

