In vivo multimodal magnetic particle imaging (MPI) with tailored magneto/optical contrast agents
- PMID: 25818431
- PMCID: PMC4379444
- DOI: 10.1016/j.biomaterials.2015.02.040
In vivo multimodal magnetic particle imaging (MPI) with tailored magneto/optical contrast agents
Abstract
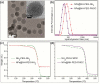
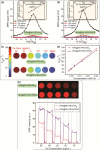
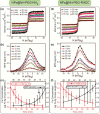
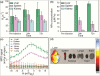
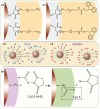
Magnetic Particle Imaging (MPI) is a novel non-invasive biomedical imaging modality that uses safe magnetite nanoparticles as tracers. Controlled synthesis of iron oxide nanoparticles (NPs) with tuned size-dependent magnetic relaxation properties is critical for the development of MPI. Additional functionalization of these NPs for other imaging modalities (e.g. MRI and fluorescent imaging) would accelerate screening of the MPI tracers based on their in vitro and in vivo performance in pre-clinical trials. Here, we conjugated two different types of poly-ethylene-glycols (NH2-PEG-NH2 and NH2-PEG-FMOC) to monodisperse carboxylated 19.7 nm NPs by amide bonding. Further, we labeled these NPs with Cy5.5 near infra-red fluorescent (NIRF) molecules. Bi-functional PEG (NH2-PEG-NH2) resulted in larger hydrodynamic size (∼98 nm vs. ∼43 nm) of the tracers, due to inter-particle crosslinking. Formation of such clusters impacted the multimodal imaging performance and pharmacokinetics of these tracers. We found that MPI signal intensity of the tracers in blood depends on their plasmatic clearance pharmacokinetics. Whole body mice MPI/MRI/NIRF, used to study the biodistribution of the injected NPs, showed primary distribution in liver and spleen. Biodistribution of tracers and their clearance pathway was further confirmed by MPI and NIRF signals from the excised organs where the Cy5.5 labeling enabled detailed anatomical mapping of the tracers.in tissue sections. These multimodal MPI tracers, combining the strengths of each imaging modality (e.g. resolution, tracer sensitivity and clinical use feasibility) pave the way for various in vitro and in vivo MPI applications.
Keywords: Biodistribution and pharmacokinetics; Magnetic nanoparticles, Magnetic Resonance Imaging; Magnetic particle imaging; Multimodal contrast agents.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Pablico-Lansigan MH, Situ SF, Samia ACS. Magnetic particle imaging: advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale. 2013;5:4040–55. - PubMed
-
- Weizenecker J, Gleich B, Rahmer J, Dahnke H, Borgert J. Three-dimensional real-time in vivo magnetic particle imaging. Physics in Medicine and Biology. 2009;54:L1–L10. - PubMed
-
- Goodwill PW, Saritas EU, Croft LR, Kim TN, Krishnan KM, Schaffer DV, et al. X-Space MPI: Magnetic Nanoparticles for Safe Medical Imaging. Advanced Materials. 2012;24:3870–7. - PubMed
-
- Gleich B, Weizenecker J. Tomographic imaging using the nonlinear response of magnetic particles. Nature. 2005;435:1214–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

