Astrocyte regulation of blood flow in the brain
- PMID: 25818565
- PMCID: PMC4448617
- DOI: 10.1101/cshperspect.a020388
Astrocyte regulation of blood flow in the brain
Abstract
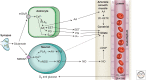
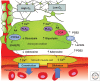
Neuronal activity results in increased blood flow in the brain, a response named functional hyperemia. Astrocytes play an important role in mediating this response. Neurotransmitters released from active neurons evoke Ca(2+) increases in astrocytes, leading to the release of vasoactive metabolites of arachidonic acid from astrocyte endfeet onto blood vessels. Synthesis of prostaglandin E2 (PGE2) and epoxyeicosatrienoic acids (EETs) dilate blood vessels, whereas 20-hydroxyeicosatetraenoic acid (20-HETE) constricts vessels. The release of K(+) from astrocyte endfeet may also contribute to vasodilation. Oxygen modulates astrocyte regulation of blood flow. Under normoxic conditions, astrocytic Ca(2+) signaling results in vasodilation, whereas under hyperoxic conditions, vasoconstriction is favored. Astrocytes also contribute to the generation of vascular tone. Tonic release of both 20-HETE and ATP from astrocytes constricts vascular smooth muscle cells, generating vessel tone. Under pathological conditions, including Alzheimer's disease and diabetic retinopathy, disruption of normal astrocyte physiology can compromise the regulation of blood flow.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Alzheimer A. 1907. Über eine eigenartige Erkrankung der Hirnrinde [About a peculiar disease of the cerebral cortex]. Allg Z Psychiat 64: 146–148.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
