HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus
- PMID: 25818806
- PMCID: PMC4380233
- DOI: 10.1038/ncomms7660
HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus
Abstract
Intracellular transport of cargos, including many viruses, involves directed movement on microtubules mediated by motor proteins. Although a number of viruses bind motors of opposing directionality, how they associate with and control these motors to accomplish directed movement remains poorly understood. Here we show that human immunodeficiency virus type 1 (HIV-1) associates with the kinesin-1 adaptor protein, Fasiculation and Elongation Factor zeta 1 (FEZ1). RNAi-mediated FEZ1 depletion blocks early infection, with virus particles exhibiting bi-directional motility but no net movement to the nucleus. Furthermore, both dynein and kinesin-1 motors are required for HIV-1 trafficking to the nucleus. Finally, the ability of exogenously expressed FEZ1 to promote early HIV-1 infection requires binding to kinesin-1. Our findings demonstrate that opposing motors both contribute to early HIV-1 movement and identify the kinesin-1 adaptor, FEZ1 as a capsid-associated host regulator of this process usurped by HIV-1 to accomplish net inward movement towards the nucleus.
Conflict of interest statement
The authors declare no conflicts of interest associated with this work.
Figures
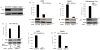



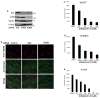
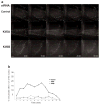

References
-
- Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14:R525–37. - PubMed
-
- Fassati A. Multiple roles of the capsid protein in the early steps of HIV-1 infection. Virus Res. 2102;170:15–24. - PubMed
-
- Arhel N, et al. Quantitative four-dimensional tracking of cytoplasmic and nuclear HIV-1 complexes. Nat Methods. 2006;3:817–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

