Post-trauma administration of the pifithrin-α oxygen analog improves histological and functional outcomes after experimental traumatic brain injury
- PMID: 25819102
- PMCID: PMC5193498
- DOI: 10.1016/j.expneurol.2015.03.015
Post-trauma administration of the pifithrin-α oxygen analog improves histological and functional outcomes after experimental traumatic brain injury
Abstract
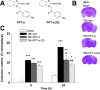
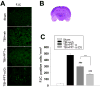
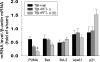
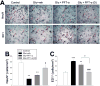
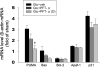
Traumatic brain injury (TBI) is a major cause of death and disability worldwide. Programmed death of neuronal cells plays a crucial role in acute and chronic neurodegeneration following TBI. The tumor suppressor protein p53, a transcription factor, has been recognized as an important regulator of apoptotic neuronal death. The p53 inactivator pifithrin-α (PFT-α) has been shown to be neuroprotective against stroke. A previous cellular study indicated that PFT-α oxygen analog (PFT-α (O)) is more stable and active than PFT-α. We aimed to investigate whether inhibition of p53 using PFT-α or PFT-α (O) would be a potential neuroprotective strategy for TBI. To evaluate whether these drugs protect against excitotoxicity in vitro, primary rat cortical cultures were challenged with glutamate (50mM) in the presence or absence of various concentrations of the p53 inhibitors PFT-α or PFT-α (O). Cell viability was estimated by LDH assay. In vivo, adult Sprague Dawley rats were subjected to controlled cortical impact (CCI, with 4m/s velocity, 2mm deformation). Five hours after injury, PFT-α or PFT-α (O) (2mg/kg, i.v.) was administered to animals. Sensory and motor functions were evaluated by behavioral tests at 24h after TBI. The p53-positive neurons were identified by double staining with cell-specific markers. Levels of mRNA encoding for p53-regulated genes (BAX, PUMA, Bcl-2 and p21) were measured by reverse transcription followed by real time-PCR from TBI animals without or with PFT-α/PFT-α (O) treatment. We found that PFT-α(O) (10 μM) enhanced neuronal survival against glutamate-induced cytotoxicity in vitro more effectively than PFT-α (10 μM). In vivo PFT-α (O) treatment enhanced functional recovery and decreased contusion volume at 24h post-injury. Neuroprotection by PFT-α (O) treatment also reduced p53-positive neurons in the cortical contusion region. In addition, p53-regulated PUMA mRNA levels at 8h were significantly reduced by PFT-α (O) administration after TBI. PFT-α (O) treatment also decreased phospho-p53 positive neurons in the cortical contusion region. Our data suggest that PFT-α (O) provided a significant reduction of cortical cell death and protected neurons from glutamate-induced excitotoxicity in vitro, as well as improved neurological functional outcome and reduced brain injury in vivo via anti-apoptotic mechanisms. The inhibition of p53-induced apoptosis by PFT-α (O) provides a useful tool to evaluate reversible apoptotic mechanisms and may develop into a novel therapeutic strategy for TBI.
Keywords: Apoptosis; Controlled cortical impact; PFT-α oxygen analog; Pifithrin-α (PFT-α); Traumatic brain injury (TBI); p53.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosure No competing financial interest exists. Conflict of interest The authors have no conflicts of interest relevant to this article to disclose.
Figures



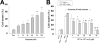
Similar articles
-
Post-traumatic administration of the p53 inactivator pifithrin-α oxygen analogue reduces hippocampal neuronal loss and improves cognitive deficits after experimental traumatic brain injury.Neurobiol Dis. 2016 Dec;96:216-226. doi: 10.1016/j.nbd.2016.08.012. Epub 2016 Aug 20. Neurobiol Dis. 2016. PMID: 27553877 Free PMC article.
-
The p53 inactivators pifithrin-μ and pifithrin-α mitigate TBI-induced neuronal damage through regulation of oxidative stress, neuroinflammation, autophagy and mitophagy.Exp Neurol. 2020 Feb;324:113135. doi: 10.1016/j.expneurol.2019.113135. Epub 2019 Nov 26. Exp Neurol. 2020. PMID: 31778663 Free PMC article.
-
Neuroprotective effects of pifithrin-α against traumatic brain injury in the striatum through suppression of neuroinflammation, oxidative stress, autophagy, and apoptosis.Sci Rep. 2018 Feb 5;8(1):2368. doi: 10.1038/s41598-018-19654-x. Sci Rep. 2018. PMID: 29402897 Free PMC article.
-
Cell death mechanisms and modulation in traumatic brain injury.Neurotherapeutics. 2010 Jan;7(1):3-12. doi: 10.1016/j.nurt.2009.10.023. Neurotherapeutics. 2010. PMID: 20129492 Free PMC article. Review.
-
Emerging treatments for traumatic brain injury.Expert Opin Emerg Drugs. 2009 Mar;14(1):67-84. doi: 10.1517/14728210902769601. Expert Opin Emerg Drugs. 2009. PMID: 19249984 Free PMC article. Review.
Cited by
-
Guanosine Protects Against Traumatic Brain Injury-Induced Functional Impairments and Neuronal Loss by Modulating Excitotoxicity, Mitochondrial Dysfunction, and Inflammation.Mol Neurobiol. 2017 Dec;54(10):7585-7596. doi: 10.1007/s12035-016-0238-z. Epub 2016 Nov 9. Mol Neurobiol. 2017. PMID: 27830534
-
The protective effect of PFTα on alcohol-induced osteonecrosis of the femoral head.Oncotarget. 2017 Jul 11;8(59):100691-100707. doi: 10.18632/oncotarget.19160. eCollection 2017 Nov 21. Oncotarget. 2017. PMID: 29246013 Free PMC article.
-
Cognitive Impairments Induced by Concussive Mild Traumatic Brain Injury in Mouse Are Ameliorated by Treatment with Phenserine via Multiple Non-Cholinergic and Cholinergic Mechanisms.PLoS One. 2016 Jun 2;11(6):e0156493. doi: 10.1371/journal.pone.0156493. eCollection 2016. PLoS One. 2016. PMID: 27254111 Free PMC article.
-
3,6'-dithiopomalidomide reduces neural loss, inflammation, behavioral deficits in brain injury and microglial activation.Elife. 2020 Jun 26;9:e54726. doi: 10.7554/eLife.54726. Elife. 2020. PMID: 32589144 Free PMC article.
-
(-)-Phenserine and Inhibiting Pre-Programmed Cell Death: In Pursuit of a Novel Intervention for Alzheimer's Disease.Curr Alzheimer Res. 2018;15(9):883-891. doi: 10.2174/1567205015666180110120026. Curr Alzheimer Res. 2018. PMID: 29318971 Free PMC article. Review.
References
-
- Andrews PJ, Piper IR, Dearden NM, Miller JD. Secondary insults during intrahospital transport of head-injured patients. Lancet. 1990;335:327–330. - PubMed
-
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005;47:29–41. - PubMed
-
- Biswas SC, Ryu E, Park C, Malagelada C, Greene LA. Puma and p53 play required roles in death evoked in a cellular model of Parkinson disease. Neurochemical research. 2005;30:839–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

