Necroptosis: The Trojan horse in cell autonomous antiviral host defense
- PMID: 25819165
- PMCID: PMC5115625
- DOI: 10.1016/j.virol.2015.03.016
Necroptosis: The Trojan horse in cell autonomous antiviral host defense
Abstract
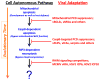
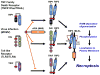
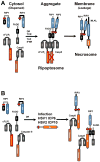
Herpesviruses suppress cell death to assure sustained infection in their natural hosts. Murine cytomegalovirus (MCMV) encodes suppressors of apoptosis as well as M45-encoded viral inhibitor of RIP activation (vIRA) to block RIP homotypic interaction motif (RHIM)-signaling and recruitment of RIP3 (also called RIPK3), to prevent necroptosis. MCMV and human cytomegalovirus encode a viral inhibitor of caspase (Casp)8 activation to block apoptosis, an activity that unleashes necroptosis. Herpes simplex virus (HSV)1 and HSV2 incorporate both RHIM and Casp8 suppression strategies within UL39-encoded ICP6 and ICP10, respectively, which are herpesvirus-conserved homologs of MCMV M45. Both HSV proteins sensitize human cells to necroptosis by blocking Casp8 activity while preventing RHIM-dependent RIP3 activation and death. In mouse cells, HSV1 ICP6 interacts with RIP3 and, surprisingly, drives necroptosis. Thus, herpesviruses have illuminated the contribution of necoptosis to host defense in the natural host as well as its potential to restrict cross-species infections in nonnatural hosts.
Keywords: FADD; Programmed necrosis; Ribonucleotide reductase; Serine/threonine protein kinase; cFLIP.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

