Pharmacovigilance in children: detecting adverse drug reactions in routine electronic healthcare records. A systematic review
- PMID: 25819310
- PMCID: PMC4594727
- DOI: 10.1111/bcp.12645
Pharmacovigilance in children: detecting adverse drug reactions in routine electronic healthcare records. A systematic review
Abstract
Aims: A systematic review of the literature published in English over 10 years was undertaken in order to describe the use of electronic healthcare data in the identification of potential adverse drug reactions (ADRs) in children.
Methods: MEDLINE and EMBASE were searched using MESH headings and text words. Titles, keywords and abstracts were checked for age <18 years, potential ADRs and electronic healthcare data. Information extracted included age, data source, pharmacovigilance method, medicines and ADRs. Studies were quality assessed.
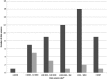
Results: From 14 804 titles, 314 had a full text review and 71 were included in the final review. Fifty were published in North America, 10 in Scandinavia. Study size ranged from less than 1000 children to more than 10 million. Sixty per cent of studies used data from one source. Comparative observational studies were most commonly reported (66.2%) with 15% using passive surveillance. Electronic healthcare data set linkage and the quality of the data source were poorly reported. ADRs were classified using the International Classification of Disease (ICD10). Multi-system reactions were most commonly studied, followed by central nervous system and mental and behavioural disorders. Vaccines were most frequently prescribed followed by corticosteroids, general anaesthetics and antidepressants.
Conclusions: Routine electronic healthcare records were increasingly reported to be used for pharmacovigilance in children. This growing and important health protection activity could be enhanced by consistent reporting of studies to improve the identification, interpretation and generalizability of the evidence base.
Keywords: adverse drug reactions; children; electronic healthcare data; systematic review.
© 2015 The British Pharmacological Society.
Figures
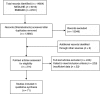
 , Population;
, Population;  , Records/Reports
, Records/Reports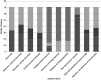
 , Not applicable;
, Not applicable;  , Not reported;
, Not reported;  , Did not meet criteria;
, Did not meet criteria;  , Partially met criteria;
, Partially met criteria;  , Met criteria
, Met criteria
Similar articles
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Systematic review of paediatric studies of adverse drug reactions from pharmacovigilance databases.Expert Opin Drug Saf. 2016 Oct;15(10):1321-8. doi: 10.1080/14740338.2016.1221921. Epub 2016 Aug 22. Expert Opin Drug Saf. 2016. PMID: 27501085
-
Understanding factors influencing the implementation of medicine risk communications by healthcare professionals in clinical practice: a systematic review using the Theoretical Domains Framework.Res Social Adm Pharm. 2024 Feb;20(2):86-98. doi: 10.1016/j.sapharm.2023.10.004. Epub 2023 Oct 18. Res Social Adm Pharm. 2024. PMID: 37978010
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review.Drug Saf. 2012 Oct 1;35(10):807-18. doi: 10.1007/BF03261977. Drug Saf. 2012. PMID: 22928729
Cited by
-
Adverse drug reactions.Br J Clin Pharmacol. 2015 Oct;80(4):613-4. doi: 10.1111/bcp.12695. Epub 2015 Sep 20. Br J Clin Pharmacol. 2015. PMID: 26388499 Free PMC article. No abstract available.
-
Effects of antidepressants on QT interval in people with mental disorders.Arch Med Sci. 2020 May 29;16(4):727-741. doi: 10.5114/aoms.2019.86928. eCollection 2020. Arch Med Sci. 2020. PMID: 32542073 Free PMC article. Review.
-
Serious Adverse Drug Reactions and Safety Signals in Children: A Nationwide Database Study.Front Pharmacol. 2020 Aug 6;11:964. doi: 10.3389/fphar.2020.00964. eCollection 2020. Front Pharmacol. 2020. PMID: 32848722 Free PMC article.
-
Monitoring Adverse Drug Events in Web Forums: Evaluation of a Pipeline and Use Case Study.J Med Internet Res. 2024 Jun 18;26:e46176. doi: 10.2196/46176. J Med Internet Res. 2024. PMID: 38888956 Free PMC article.
-
Multidisciplinary approach to improve spontaneous ADR reporting in the pediatric outpatient setting: a single-institute experience in Korea.Springerplus. 2016 Aug 30;5(1):1435. doi: 10.1186/s40064-016-3151-z. eCollection 2016. Springerplus. 2016. PMID: 27652011 Free PMC article.
References
-
- Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, Hebert L, Newhouse JP, Weiler PC, Hiatt H. The nature of adverse events in hospitalized patients-results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–84. - PubMed
-
- Thomas EJ, Studdert DM, Burstin HR, Orav EJ, Zeena T, Williams EJ, Howard KM, Weiler PC, Brennan TA. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–71. - PubMed
-
- WHO. 2002. Safety of Medicines - A Guide to Detecting and Reporting Adverse Drug Reactions - Why Health Professionals Need to Take Action. WHO/EDM/QSM/2002.2.. Geneva, World Health Organization.
-
- Impicciatore P, Mohn A, Chiarelli F, Pandolfini C, Bonati M. Adverse drug reactions to off-label drugs on a paediatric ward: an Italian prospective pilot study. Paediatric and Perinatal Drug Therapy. 2002;5:19–24.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

