Design, synthesis and biological evaluation of a highly-potent and cancer cell selective folate-taxoid conjugate
- PMID: 25819334
- PMCID: PMC4398638
- DOI: 10.1016/j.bmc.2015.02.057
Design, synthesis and biological evaluation of a highly-potent and cancer cell selective folate-taxoid conjugate
Abstract
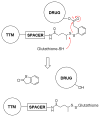
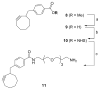
The folate receptor (FR) has been widely recognized as an excellent target for the tumor-selective delivery of cytotoxic agents, and four folate-drug conjugates have entered clinical evaluations for the treatment of solid tumors to date. However, most of these conjugates required structural modification of the cytotoxic warheads in order to achieve efficient drug release from the linkers. We designed and constructed a novel folate conjugate of a highly-potent next-generation taxoid, SB-T-1214, by exploiting bioorthogonal Cu-free 'click' chemistry. The synthesis was highly convergent and required no HPLC purification to obtain the final folate-taxoid conjugate 1. Conjugate 1 demonstrated highly FR-specific potency (IC₅₀ 2.1-3.5 nM) against a panel of cancer cell lines, with a >1000-fold decrease in cytotoxicity against normal human cells (IC₅₀>5000 nM). The remarkable potency and selectivity of conjugate 1 can be attributed to highly FR-specific receptor-mediated endocytosis as well as efficient release of the unmodified cytotoxic warhead using a mechanism-based self-immolative linker.
Keywords: Anticancer agent; Drug conjugate; Folic acid; Taxoid; Tumor-targeted.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Folate-vinca alkaloid conjugates for cancer therapy: a structure-activity relationship.Bioconjug Chem. 2014 Mar 19;25(3):560-8. doi: 10.1021/bc400441s. Epub 2014 Mar 11. Bioconjug Chem. 2014. PMID: 24564229
-
Targeted Delivery of Cabazitaxel by Conjugation to Albumin-PEG-folate Nanoparticles Using a Cysteine-acrylate Linker and Simple Synthesis Conditions.Curr Drug Deliv. 2017;14(8):1120-1129. doi: 10.2174/1567201814666161122150302. Curr Drug Deliv. 2017. PMID: 27875950
-
Guided molecular missiles for tumor-targeting chemotherapy--case studies using the second-generation taxoids as warheads.Acc Chem Res. 2008 Jan;41(1):108-19. doi: 10.1021/ar700093f. Epub 2007 Jul 31. Acc Chem Res. 2008. PMID: 17663526 Review.
-
Mechanism-based tumor-targeting drug delivery system. Validation of efficient vitamin receptor-mediated endocytosis and drug release.Bioconjug Chem. 2010 May 19;21(5):979-87. doi: 10.1021/bc9005656. Bioconjug Chem. 2010. PMID: 20429547 Free PMC article.
-
Targeted cancer therapy: conferring specificity to cytotoxic drugs.Acc Chem Res. 2008 Jan;41(1):98-107. doi: 10.1021/ar700108g. Epub 2007 Aug 18. Acc Chem Res. 2008. PMID: 17705444 Review.
Cited by
-
Cellular delivery of dinucleotides by conjugation with small molecules: targeting translation initiation for anticancer applications.Chem Sci. 2021 Jun 29;12(30):10242-10251. doi: 10.1039/d1sc02143e. eCollection 2021 Aug 4. Chem Sci. 2021. PMID: 34377411 Free PMC article.
-
Strategic Incorporation of Fluorine into Taxoid Anticancer Agents for Medicinal Chemistry and Chemical Biology Studies.J Fluor Chem. 2017 Jun;198:10-23. doi: 10.1016/j.jfluchem.2016.12.016. Epub 2017 Jan 3. J Fluor Chem. 2017. PMID: 28824201 Free PMC article.
-
Advances in targeting the folate receptor in the treatment/imaging of cancers.Chem Sci. 2017 Dec 18;9(4):790-810. doi: 10.1039/c7sc04004k. eCollection 2018 Jan 28. Chem Sci. 2017. PMID: 29675145 Free PMC article. Review.
-
Active Targeted Nanoformulations via Folate Receptors: State of the Art and Future Perspectives.Pharmaceutics. 2021 Dec 22;14(1):14. doi: 10.3390/pharmaceutics14010014. Pharmaceutics. 2021. PMID: 35056911 Free PMC article. Review.
-
Taxane anticancer agents: a patent perspective.Expert Opin Ther Pat. 2016;26(1):1-20. doi: 10.1517/13543776.2016.1111872. Epub 2015 Dec 10. Expert Opin Ther Pat. 2016. PMID: 26651178 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

