Optic nerve head biomechanics in aging and disease
- PMID: 25819451
- PMCID: PMC4379445
- DOI: 10.1016/j.exer.2015.02.011
Optic nerve head biomechanics in aging and disease
Abstract
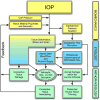
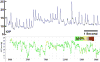
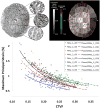
This nontechnical review is focused upon educating the reader on optic nerve head biomechanics in both aging and disease along two main themes: what is known about how mechanical forces and the resulting deformations are distributed in the posterior pole and ONH (biomechanics) and what is known about how the living system responds to those deformations (mechanobiology). We focus on how ONH responds to IOP elevations as a structural system, insofar as the acute mechanical response of the lamina cribrosa is confounded with the responses of the peripapillary sclera, prelaminar neural tissues, and retrolaminar optic nerve. We discuss the biomechanical basis for IOP-driven changes in connective tissues, blood flow, and cellular responses. We use glaucoma as the primary framework to present the important aspects of ONH biomechanics in aging and disease, as ONH biomechanics, aging, and the posterior pole extracellular matrix (ECM) are thought to be centrally involved in glaucoma susceptibility, onset and progression.
Keywords: Biomechanics; Glaucoma; ONH; Optic nerve head; Sclera.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Agapova OA, Kaufman PL, Lucarelli MJ, Gabelt BT, Hernandez MR. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003;967(1-2):132–143. - PubMed
-
- Agoumi Y, Sharpe GP, Hutchison DM, Nicolela MT, Artes PH, Chauhan BC. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology. 2011;118(1):52–59. - PubMed
-
- Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106(1-2):1–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

