Regulation of corneal stroma extracellular matrix assembly
- PMID: 25819456
- PMCID: PMC4379422
- DOI: 10.1016/j.exer.2014.08.001
Regulation of corneal stroma extracellular matrix assembly
Abstract
The transparent cornea is the major refractive element of the eye. A finely controlled assembly of the stromal extracellular matrix is critical to corneal function, as well as in establishing the appropriate mechanical stability required to maintain corneal shape and curvature. In the stroma, homogeneous, small diameter collagen fibrils, regularly packed with a highly ordered hierarchical organization, are essential for function. This review focuses on corneal stroma assembly and the regulation of collagen fibrillogenesis. Corneal collagen fibrillogenesis involves multiple molecules interacting in sequential steps, as well as interactions between keratocytes and stroma matrix components. The stroma has the highest collagen V:I ratio in the body. Collagen V regulates the nucleation of protofibril assembly, thus controlling the number of fibrils and assembly of smaller diameter fibrils in the stroma. The corneal stroma is also enriched in small leucine-rich proteoglycans (SLRPs) that cooperate in a temporal and spatial manner to regulate linear and lateral collagen fibril growth. In addition, the fibril-associated collagens (FACITs) such as collagen XII and collagen XIV have roles in the regulation of fibril packing and inter-lamellar interactions. A communicating keratocyte network contributes to the overall and long-range regulation of stromal extracellular matrix assembly, by creating micro-domains where the sequential steps in stromal matrix assembly are controlled. Keratocytes control the synthesis of extracellular matrix components, which interact with the keratocytes dynamically to coordinate the regulatory steps into a cohesive process. Mutations or deficiencies in stromal regulatory molecules result in altered interactions and deficiencies in both transparency and refraction, leading to corneal stroma pathobiology such as stromal dystrophies, cornea plana and keratoconus.
Keywords: collagens; corneal stroma; extracellular matrix; fibrillogenesis; small leucine-rich proteoglycans; stromal organization.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

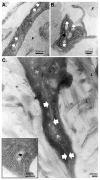
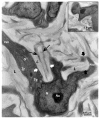



References
-
- Akimoto Y, Yamakawa N, Furukawa K, Kimata K, Kawakami H, Hirano H. Changes in distribution of the long form of type XII collagen during chicken corneal development. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2002;50:851–862. - PubMed
-
- Anderson S, SundarRaj S, Fite D, Wessel H, SundarRaj N. Developmentally regulated appearance of spliced variants of type XII collagen in the cornea. Invest Ophthalmol Vis Sci. 2000;41:55–63. - PubMed
-
- Anseth A. Glycosaminoglycans in corneal regeneration. Experimental eye research. 1961;1:122–127. - PubMed
-
- Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228–244. - PubMed
-
- Beecher N, Carlson C, Allen BR, Kipchumba R, Conrad GW, Meek KM, Quantock AJ. An x-ray diffraction study of corneal structure in mimecan-deficient mice. Invest Ophthalmol Vis Sci. 2005;46:4046–4049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

