Role of tyrosine-sulfated proteins in retinal structure and function
- PMID: 25819460
- PMCID: PMC4379419
- DOI: 10.1016/j.exer.2014.07.007
Role of tyrosine-sulfated proteins in retinal structure and function
Abstract
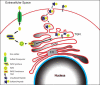
The extracellular matrix (ECM) plays a significant role in cellular and retinal health. The study of retinal tyrosine-sulfated proteins is an important first step toward understanding the role of ECM in retinal health and diseases. These secreted proteins are members of the retinal ECM. Tyrosine sulfation was shown to be necessary for the development of proper retinal structure and function. The importance of tyrosine sulfation is further demonstrated by the evolutionary presence of tyrosylprotein sulfotransferases, enzymes that catalyze proteins' tyrosine sulfation, and the compensatory abilities of these enzymes. Research has identified four tyrosine-sulfated retinal proteins: fibulin 2, vitronectin, complement factor H (CFH), and opticin. Vitronectin and CFH regulate the activation of the complement system and are involved in the etiology of some cases of age-related macular degeneration. Analysis of the role of tyrosine sulfation in fibulin function showed that sulfation influences the protein's ability to regulate growth and migration. Although opticin was recently shown to exhibit anti-angiogenic properties, it is not yet determined what role sulfation plays in that function. Future studies focusing on identifying all of the tyrosine-sulfated retinal proteins would be instrumental in determining the impact of sulfation on retinal protein function in retinal homeostasis and diseases.
Keywords: extracellular matrix; retina; retinal diseases; tyrosine sulfation.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
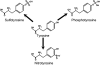
References
-
- Antonsson P, Heinegard D, Oldberg A. Posttranslational modifications of fibromodulin. J.Biol.Chem. 1991;266:16859–61. - PubMed
-
- Arruda VR, Hagstrom JN, Deitch J, Heiman-Patterson T, Camire RM, Chu K, Fields PA, Herzog RW, Couto LB, Larson PJ, High KA. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001;97:130–8. - PubMed
-
- Boehncke W-H, Schön M. Interfering with leukocyte rolling-a promising therapeutic approach in inflammatory skin disorders? Trends Pharmacol. Sci. 2003;24:49–52. - PubMed
-
- Bonomi M, Busnelli M, Persani L, Vassart G, Costagliola S. Structural differences in the hinge region of the glycoprotein hormone receptors: evidence from the sulfated tyrosine residues. Mol.Endocrinol. 2006;20:3351–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

