Treatment of spontaneous preterm labour with retosiban: a phase 2 proof-of-concept study
- PMID: 25819462
- PMCID: PMC4594710
- DOI: 10.1111/bcp.12646
Treatment of spontaneous preterm labour with retosiban: a phase 2 proof-of-concept study
Erratum in
-
Corrigendum.Br J Clin Pharmacol. 2015 Sep;80(3):609. doi: 10.1111/bcp.12694. Epub 2015 Jul 6. Br J Clin Pharmacol. 2015. PMID: 26307341 Free PMC article. No abstract available.
Abstract
Aim: The aim was to investigate the efficacy and safety of intravenous retosiban in women with spontaneous preterm labour.
Methods: This was a randomized, double-blind, placebo-controlled, phase 2 trial. Retosiban was administered intravenously for 48 h to women in spontaneous preterm labour between 30(0/7) and 35(6/7) weeks' gestation with an uncomplicated singleton pregnancy in an in-patient obstetric unit. Outcome measures were uterine quiescence (primary endpoint), days to delivery, preterm delivery and safety.
Results: Uterine quiescence was achieved in 62% of women who received retosiban (n = 30) compared with 41% who received placebo (n = 34). The relative risk (RR) was 1.53 (95% credible interval [CrI] 0.98, 2.48; NS). Retosiban resulted in a significant increase in time to delivery compared with placebo (mean difference 8.2 days, 95% CrI 2.7, 13.74). This difference was consistent across all gestational ages. The proportion of preterm births in the retosiban and placebo groups was 18.7% (95% CrI 7.4%, 33.7%) and 47.2% (95% CrI 31.4%, 63.4%), respectively. The RR of preterm birth in women treated with retosiban was 0.38 (95% CrI 0.15, 0.81). There were no deliveries within 7 days in the retosiban group, but there were six (17.6%) births in the placebo group. The maternal, fetal and neonatal adverse events were comparable in the retosiban and placebo groups.
Conclusions: Intravenous administration of retosiban in women with spontaneous preterm labour was associated with a greater than 1 week increase in time to delivery compared with placebo, a significant reduction in preterm deliveries, a non-significant increase in uterine quiescence and a favourable safety profile.
Keywords: preterm birth; preterm labour; proof-of-concept study; retosiban; uterine quiescence.
© 2015 The British Pharmacological Society.
Figures
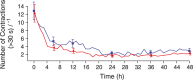
 , retosiban, (n = 30);
, retosiban, (n = 30);  , placebo (n = 34)
, placebo (n = 34)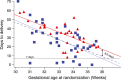
 , retosiban;
, retosiban;  , placebo
, placebo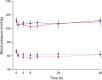
 , retosiban, systolic;
, retosiban, systolic;  , placebo, systolic;
, placebo, systolic;  , retosiban, diastolic;
, retosiban, diastolic;  , placebo, diastolic
, placebo, diastolic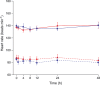
 , retosiban, fetal;
, retosiban, fetal;  , placebo, fetal;
, placebo, fetal;  , retosiban, mother;
, retosiban, mother;  , placebo, mother
, placebo, motherReferences
-
- American College of Obstetrics and Gynecologists, Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 127: Management of preterm labour. Obstet Gynecol. 2012;119:1308–17. - PubMed
-
- Engle WA, Tomashek KM, Wallman C. ‘Late-preterm’ infants: a population at risk. Pediatrics. 2007;120:1390–401. - PubMed
-
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. - PubMed
-
- Royal College of Obstetricians and Gynaecologists. 2011. Tocolytic drugs for women in preterm labour. Green-top Guideline No 1B, February [online]. Available at http://www.rcog.org.uk/womens-health/clinical-guidance/tocolytic-drugs-w... (last accessed 24 July 2014)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

