PLETHORA Genes Control Regeneration by a Two-Step Mechanism
- PMID: 25819565
- PMCID: PMC4829346
- DOI: 10.1016/j.cub.2015.02.022
PLETHORA Genes Control Regeneration by a Two-Step Mechanism
Abstract
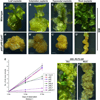
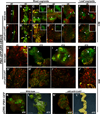
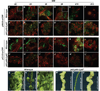
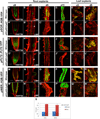
Regeneration, a remarkable example of developmental plasticity displayed by both plants and animals, involves successive developmental events driven in response to environmental cues. Despite decades of study on the ability of the plant tissues to regenerate a complete fertile shoot system after inductive cues, the mechanisms by which cells acquire pluripotency and subsequently regenerate complete organs remain unknown. Here, we show that three PLETHORA (PLT) genes, PLT3, PLT5, and PLT7, regulate de novo shoot regeneration in Arabidopsis by controlling two distinct developmental events. Cumulative loss of function of these three genes causes the intermediate cell mass, callus, to be incompetent to form shoot progenitors, whereas induction of PLT5 or PLT7 can render shoot regeneration hormone-independent. We further show that PLT3, PLT5, and PLT7 establish pluripotency by activating root stem cell regulators PLT1 and PLT2, as reconstitution of either PLT1 or PLT2 in the plt3; plt5-2; plt7 mutant re-established the competence to regenerate shoot progenitor cells but did not lead to the completion of shoot regeneration. PLT3, PLT5, and PLT7 additionally regulate and require the shoot-promoting factor CUP-SHAPED COTYLEDON2 (CUC2) to complete the shoot-formation program. Our findings uncouple the acquisition of competence to regenerate shoot progenitor cells from completion of shoot formation, indicating a two-step mechanism of de novo shoot regeneration that operates in all tested plant tissues irrespective of their origin. Our studies reveal intermediate developmental phases of regeneration and provide a deeper understanding into the mechanistic basis of regeneration.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
-
- Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development (Cambridge, England) 2007;134:3539–3548. - PubMed
-
- Atta R, Laurens L, Boucheron-Dubuisson E, Guivarc'h A, Carnero E, Giraudat-Pautot V, Rech P, Chriqui D. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. The Plant journal : for cell and molecular biology. 2009;57:626–644. - PubMed
-
- Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symposia of the Society for Experimental Biology. 1957;11:118–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

