The RpoE Stress Response Pathway Mediates Reduction of the Virulence of Enteropathogenic Escherichia coli by Zinc
- PMID: 25819956
- PMCID: PMC4421060
- DOI: 10.1128/AEM.00507-15
The RpoE Stress Response Pathway Mediates Reduction of the Virulence of Enteropathogenic Escherichia coli by Zinc
Abstract
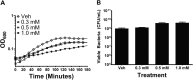
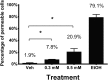
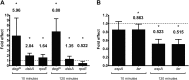
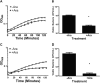
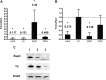
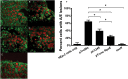
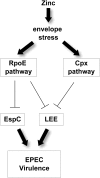
Zinc supplements are an effective clinical treatment for infantile diarrheal disease caused by enteric pathogens. Previous studies demonstrated that zinc acts on enteropathogenic Escherichia coli (EPEC) bacteria directly to suppress several virulence-related genes at a concentration that can be achieved by oral delivery of dietary zinc supplements. Our in vitro studies showed that a micromolar concentration of zinc induced the envelope stress response and suppressed virulence in EPEC, providing a possible mechanistic explanation for zinc's therapeutic action. In this report, we investigated the molecular and physiological changes in EPEC induced by zinc. We found that micromolar concentrations of zinc reduced the bacterial growth rate without affecting viability. We observed increased membrane permeability caused by zinc. Zinc upregulated the RpoE-dependent envelope stress response pathway and suppressed EPEC virulence gene expression. RpoE alone was sufficient to inhibit virulence factor expression and to attenuate attaching and effacing lesion formation on human host cells. By mutational analysis we demonstrate that the DNA-binding motif of RpoE is necessary for suppression of the LEE1, but not the LEE4, operon. Predictably, inhibition of the RpoE-mediated envelope stress response in combination with micromolar concentrations of zinc reduced EPEC viability. In conclusion, zinc induces the RpoE and stress response pathways in EPEC, and the alternate sigma factor RpoE downregulates EPEC LEE and non-LEE virulence genes by multiple mechanisms.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Hfq reduces envelope stress by controlling expression of envelope-localized proteins and protein complexes in enteropathogenic Escherichia coli.Mol Microbiol. 2014 May;92(4):681-97. doi: 10.1111/mmi.12581. Epub 2014 Apr 15. Mol Microbiol. 2014. PMID: 24628810
-
Zinc-induced envelope stress diminishes type III secretion in enteropathogenic Escherichia coli.BMC Microbiol. 2012 Jun 24;12:123. doi: 10.1186/1471-2180-12-123. BMC Microbiol. 2012. PMID: 22727253 Free PMC article.
-
Effect of zinc in enteropathogenic Escherichia coli infection.Infect Immun. 2007 Dec;75(12):5974-84. doi: 10.1128/IAI.00750-07. Epub 2007 Sep 17. Infect Immun. 2007. PMID: 17875638 Free PMC article.
-
Acid-happy: Survival and recovery of enteropathogenic Escherichia coli (EPEC) in simulated gastric fluid.Microb Pathog. 2019 Mar;128:396-404. doi: 10.1016/j.micpath.2019.01.022. Epub 2019 Jan 17. Microb Pathog. 2019. PMID: 30660737
-
Pathophysiology of enteropathogenic Escherichia coli during a host infection.J Vet Sci. 2022 Mar;23(2):e28. doi: 10.4142/jvs.21160. Epub 2022 Jan 27. J Vet Sci. 2022. PMID: 35187883 Free PMC article. Review.
Cited by
-
Characterization of the Mode of Action of Aurodox, a Type III Secretion System Inhibitor from Streptomyces goldiniensis.Infect Immun. 2019 Jan 24;87(2):e00595-18. doi: 10.1128/IAI.00595-18. Print 2019 Feb. Infect Immun. 2019. PMID: 30455200 Free PMC article.
-
Nutritional immunity: the battle for nutrient metals at the host-pathogen interface.Nat Rev Microbiol. 2022 Nov;20(11):657-670. doi: 10.1038/s41579-022-00745-6. Epub 2022 May 31. Nat Rev Microbiol. 2022. PMID: 35641670 Free PMC article. Review.
-
PerC Manipulates Metabolism and Surface Antigens in Enteropathogenic Escherichia coli.Front Cell Infect Microbiol. 2017 Feb 7;7:32. doi: 10.3389/fcimb.2017.00032. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28224117 Free PMC article.
-
High-Zinc Supplementation of Weaned Piglets Affects Frequencies of Virulence and Bacteriocin Associated Genes Among Intestinal Escherichia coli Populations.Front Vet Sci. 2020 Dec 16;7:614513. doi: 10.3389/fvets.2020.614513. eCollection 2020. Front Vet Sci. 2020. PMID: 33392299 Free PMC article.
-
Micronutrients: A double-edged sword in microbial-induced gastric carcinogenesis.Trends Cancer. 2015 Oct 1;1(2):136-144. doi: 10.1016/j.trecan.2015.07.002. Trends Cancer. 2015. PMID: 26623443 Free PMC article.
References
-
- Shankar AH, Prasad AS. 1998. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 68:447S–463S. - PubMed
-
- Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. 2013. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 8:e72788. doi:10.1371/journal.pone.0072788. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

