Binding-induced, turn-on fluorescence of the EGFR/ERBB kinase inhibitor, lapatinib
- PMID: 25820099
- PMCID: PMC4548262
- DOI: 10.1039/c5ob00239g
Binding-induced, turn-on fluorescence of the EGFR/ERBB kinase inhibitor, lapatinib
Abstract
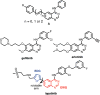
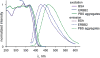
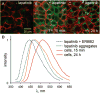
We report the photophysical properties, binding-induced turn-on emission, and fluorescence imaging of the cellular uptake and distribution of lapatinib, an EGFR/ERBB inhibitor. Lapatinib, a type II, i.e. inactive state, inhibitor that targets the ATP binding pocket of the EGFR family of receptor tyrosine kinases. DFT calculations predict that the 6-furanylquinazoline core of lapatinib should exhibit an excited state with charge transfer character and an S0 to S1 transition energy of 3.4 eV. Absorption confirms an optical transition in the near UV to violet, while fluorescence spectroscopy shows that photoemission is highly sensitive to solvent polarity. The hydrophobicity of lapatinib leads to fluorescent aggregates in solution, however, binding to the lipid-carrier protein, BSA or to the kinase domain of ERBB2, produces spectroscopically distinct photoemission. Confocal fluorescence microscopy imaging of lapatinib uptake in ERBB2-overexpressing MCF7 and BT474 cells reveals pools of intracellular inhibitor with emission profiles consistent with aggregated lapatinib.
Figures
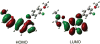
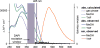

References
-
- Wilhelmson ML. Q Rev Biophys. 2010;43:159. - PubMed
-
- Yanai T, Tew DP, Handy NC. Chem Phys Lett. 2004;393:51.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

