Mapping the zoonotic niche of Marburg virus disease in Africa
- PMID: 25820266
- PMCID: PMC4447827
- DOI: 10.1093/trstmh/trv024
Mapping the zoonotic niche of Marburg virus disease in Africa
Abstract
Background: Marburg virus disease (MVD) describes a viral haemorrhagic fever responsible for a number of outbreaks across eastern and southern Africa. It is a zoonotic disease, with the Egyptian rousette (Rousettus aegyptiacus) identified as a reservoir host. Infection is suspected to result from contact between this reservoir and human populations, with occasional secondary human-to-human transmission.
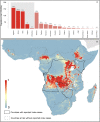
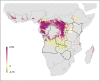
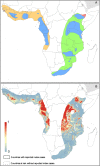
Methods: Index cases of previous human outbreaks were identified and reports of infection in animals recorded. These data were modelled within a species distribution modelling framework in order to generate a probabilistic surface of zoonotic transmission potential of MVD across sub-Saharan Africa.
Results: Areas suitable for zoonotic transmission of MVD are predicted in 27 countries inhabited by 105 million people. Regions are suggested for exploratory surveys to better characterise the geographical distribution of the disease, as well as for directing efforts to communicate the risk of practices enhancing zoonotic contact.
Conclusions: These maps can inform future contingency and preparedness strategies for MVD control, especially where secondary transmission is a risk. Coupling this risk map with patient travel histories could be used to guide the differential diagnosis of highly transmissible pathogens, enabling more rapid response to outbreaks of haemorrhagic fever.
Keywords: Boosted regression trees; Filovirus; Marburg virus disease; Rousettus aegyptiacus; Species distribution models; Viral haemorrhagic fever.
© The Author 2015. Published by Oxford University Press on behalf of Royal Society of Tropical Medicine and Hygiene.
Figures



References
-
- Slenczka WG. The Marburg virus outbreak of 1967 and subsequent episodes. Curr Top Microbiol Immunol 1999;235:49–75. - PubMed
-
- Siegert R, Shu H-L, Slenczka W, et al. On the eitology of an unknown infection originating in monkeys [in German]. Dtsch med Wochenschr 1967;92:2341–3. - PubMed
-
- Conrad JL, Isaacson M, Smith EB, et al. Epidemiologic investigation of Marburg virus disease, Southern Africa, 1975. Am J Trop Med Hyg 1978;27:1210–5. - PubMed
-
- Bausch DG, Nichol ST, Muyembe-Tamfum JJ, et al. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N Engl J Med 2006;355:909–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

