Coil combination for receive array spectroscopy: Are data-driven methods superior to methods using computed field maps?
- PMID: 25820303
- PMCID: PMC4744755
- DOI: 10.1002/mrm.25618
Coil combination for receive array spectroscopy: Are data-driven methods superior to methods using computed field maps?
Abstract
Purpose: Combining spectra from receive arrays, particularly X-nuclear spectra with low signal-to-noise ratios (SNRs), is challenging. We test whether data-driven combination methods are better than using computed coil sensitivities.
Theory: Several combination algorithms are recast into the notation of Roemer's classic formula, showing that they differ primarily in their estimation of coil receive sensitivities. This viewpoint reveals two extensions of the whitened singular-value decomposition (WSVD) algorithm, using temporal or temporal + spatial apodization to improve the coil sensitivities, and thus the combined spectral SNR.
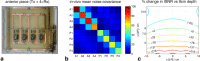
Methods: Radiofrequency fields from an array were simulated and used to make synthetic spectra. These were combined with 10 algorithms. The combined spectra were then assessed in terms of their SNR. Validation used phantoms and cardiac (31) P spectra from five subjects at 3T.
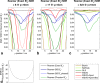
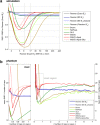
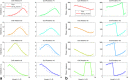
Results: Combined spectral SNRs from simulations, phantoms, and humans showed the same trends. In phantoms, the combined SNR using computed coil sensitivities was lower than with WSVD combination whenever the WSVD SNR was >14 (or >11 with temporal apodization, or >9 with temporal + spatial apodization). These new apodized WSVD methods gave higher SNRs than other data-driven methods.
Conclusion: In the human torso, at frequencies ≥49 MHz, data-driven combination is preferable to using computed coil sensitivities. Magn Reson, 2015. © 2015 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. Magn Reson Med 75:473-487, 2016. © 2015 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Keywords: MR spectroscopy; WSVD; WSVD+Apod; WSVD+Apod+Blur; adaptive combination theory; array; coil combination.
© 2015 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Figures




References
-
- De Graaf RA. In vivo NMR Spectroscopy: Principles and Techniques. Chichester, UK: John Wiley & Sons; 2007. 570 p.
-
- Bottomley PA. NMR Spectroscopy of the Human Heart In: Harris RK, Wasylishen RE, editors. Encyclopedia of Magnetic Resonance. Chichester, UK: John Wiley; 2009. doi 10.1002/9780470034590.emrstm0345.pub2. - DOI
-
- Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR Phased Array. Magn Reson Med 1990; 16: 192–225. - PubMed
-
- Wright SM, Wald LL. Theory and application of array coils in MR spectroscopy. NMR Biomed 1997; 10: 394–410. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

