Under-reporting of adverse drug reactions: a challenge for pharmacovigilance in India
- PMID: 25821314
- PMCID: PMC4375822
- DOI: 10.4103/0253-7613.150344
Under-reporting of adverse drug reactions: a challenge for pharmacovigilance in India
Abstract
Aim: The aim was to evaluate the extent and factors responsible for underreporting (UR) of adverse drug reactions (ADRs) in India.
Materials and methods: A retrospective observational, cross-sectional prospective questionnaire-based analysis was undertaken to evaluate the extent and factors for UR of ADRs in pharmacovigilance.
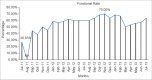
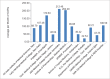
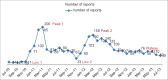
Results: At the time, this report was prepared, 90 ADR Monitoring Centers (AMC) were operational in India. Indian AMC functional rate was 56.45%. The average number of Individual Case Safety Reports reported by our center via VigiFlow per month was 48.038. In a period of the 3 years the total number of ADRs reported was 3024. The average number of reports per month was 80.08. Active surveillance versus spontaneous reporting contributed 66.13% versus 33.86% of the total ADRs (P < 0.0001). Outpatient Department (OPD) contribution was 76.05% and indoor contribution was 23.94% of total reports (P < 0.0001). Department of Medicine (33%), followed by oncology (19.27%) and chest disease (13.49%) contributed maximally. The contribution of Pharmacology ADR monitoring OPD was 16.20%. Eye, ear, nose and throat and surgery, private Medical Colleges, hospitals in periphery, sub-district and district contributed no ADRs. ADR detection rates by clinical presentation, biochemical investigation and diagnostic tools were 84.33%, 14.57%, and 1.09% respectively (P < 0.0001). Reporting by postgraduate, registrars, consultants and nurses were 72.65%, 6.58%, 16.56% and 4.19% respectively (P < 0.0001). PG students in Pharmacology contributed an average number of 5.61 ADR reports/month. The lack of knowledge and awareness about Pharmacovigilance Programme of India (PvPI), lethargy, indifference, insecurity, complacency, workload, lack of training were the common factors responsible for UR. Major academic activity, exams, thesis and synopsis submission time influenced reporting of ADRs by postgraduate students.
Conclusion: UR is a matter of concern PvPI. Multiple interventions are needed to improve ADR reporting.
Keywords: Adverse drug reaction; pharmacovigilance; spontaneous reporting; under reporting.
Conflict of interest statement
Figures
References
-
- Pushkin R, Frassetto L, Tsourounis C, Segal ES, Kim S. Improving the reporting of adverse drug reactions in the hospital setting. Postgrad Med. 2010;122:154–64. - PubMed
-
- Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernández-Díaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf. 2007;30:1073–82. - PubMed
-
- Hazell L, Shakir SA. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006;29:385–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

