Helicobacter pylori outer membrane vesicle proteins induce human eosinophil degranulation via a β2 Integrin CD11/CD18- and ICAM-1-dependent mechanism
- PMID: 25821353
- PMCID: PMC4364020
- DOI: 10.1155/2015/301716
Helicobacter pylori outer membrane vesicle proteins induce human eosinophil degranulation via a β2 Integrin CD11/CD18- and ICAM-1-dependent mechanism
Abstract
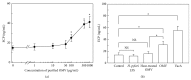
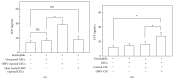
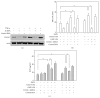
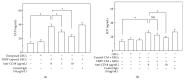
Eosinophil cationic protein (ECP), a cytotoxic protein contained in eosinophils granules, can contribute to various inflammatory responses. Although Helicobacter pylori infection increases infiltration of eosinophils, the mechanisms of eosinophil degranulation by H. pylori infection are largely unknown. The goal of this study was to investigate the role of H. pylori outer membrane vesicles (OMVs) in modulating eosinophil degranulation. We found that eosinophils treated with H. pylori OMVs released significantly more ECP compared with untreated controls. In addition, eosinophils cocultured with OMV-preexposed primary gastric epithelial cells exhibited significantly increased ECP release. Similarly, eosinophils cocultured with culture supernatant (CM) from primary gastric epithelial cells exposed to OMVs (OMV-CM) released significantly higher amounts of ECP compared with eosinophils cocultured with CM from unexposed control cells. Furthermore, OMVs and OMV-CM both induced the upregulation of ICAM-1 on gastric epithelial cells and β2 integrin CD11b on eosinophils. In addition, both transduction of ICAM-1 shRNA into gastric epithelial cells and treatment with neutralizing mAbs to CD18 significantly decreased OMV-mediated or OMV-CM-mediated release of ECP. These results suggest that the eosinophil degranulation response to H. pylori OMVs occurs via a mechanism that is dependent on both β2 integrin CD11/CD18 and ICAM-1.
Figures



Similar articles
-
Helicobacter pylori Outer Membrane Vesicles: Biogenesis, Composition, and Biological Functions.Int J Biol Sci. 2024 Jul 15;20(10):4029-4043. doi: 10.7150/ijbs.94156. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113715 Free PMC article. Review.
-
Respiratory syncytial virus-infected pulmonary epithelial cells induce eosinophil degranulation by a CD18-mediated mechanism.J Immunol. 1998 May 15;160(10):4889-95. J Immunol. 1998. PMID: 9590236
-
Eosinophil infiltration, gastric juice and serum eosinophil cationic protein levels in Helicobacter pylori-associated chronic gastritis and gastric ulcer.Mediators Inflamm. 2004 Dec;13(5-6):369-72. doi: 10.1155/S0962935104000559. Mediators Inflamm. 2004. PMID: 15770055 Free PMC article.
-
A CD18/ICAM-1-dependent pathway mediates eosinophil adhesion to human bronchial epithelial cells.Am J Respir Cell Mol Biol. 1998 Sep;19(3):408-18. doi: 10.1165/ajrcmb.19.3.3179. Am J Respir Cell Mol Biol. 1998. PMID: 9730868
-
[The roles of adhesion molecules, cytokines, and chemokines in eosinophil activation during allergic inflammation].Nihon Kyobu Shikkan Gakkai Zasshi. 1996 Dec;34 Suppl:116-20. Nihon Kyobu Shikkan Gakkai Zasshi. 1996. PMID: 9216199 Review. Japanese.
Cited by
-
A Player and Coordinator: The Versatile Roles of Eosinophils in the Immune System.Transfus Med Hemother. 2016 Mar;43(2):96-108. doi: 10.1159/000445215. Epub 2016 Mar 18. Transfus Med Hemother. 2016. PMID: 27226792 Free PMC article. Review.
-
Eosinophils as drivers of bacterial immunomodulation and persistence.Infect Immun. 2024 Sep 10;92(9):e0017524. doi: 10.1128/iai.00175-24. Epub 2024 Jul 15. Infect Immun. 2024. PMID: 39007622 Free PMC article. Review.
-
Helicobacter pylori Outer Membrane Vesicles: Biogenesis, Composition, and Biological Functions.Int J Biol Sci. 2024 Jul 15;20(10):4029-4043. doi: 10.7150/ijbs.94156. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113715 Free PMC article. Review.
-
Helicobacter pylori-Derived Outer Membrane Vesicles (OMVs): Role in Bacterial Pathogenesis?Microorganisms. 2020 Aug 31;8(9):1328. doi: 10.3390/microorganisms8091328. Microorganisms. 2020. PMID: 32878302 Free PMC article. Review.
-
Bacterial outer membrane vesicles, a potential vaccine candidate in interactions with host cells based.Diagn Pathol. 2018 Dec 11;13(1):95. doi: 10.1186/s13000-018-0768-y. Diagn Pathol. 2018. PMID: 30537996 Free PMC article. Review.
References
-
- Genta R. M., Lew G. M., Graham D. Y. Changes in the gastric mucosa following eradication of Helicobacter pylori . Modern Pathology. 1993;6(3):281–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

