The role of innate immunity receptors in the pathogenesis of inflammatory bowel disease
- PMID: 25821356
- PMCID: PMC4364059
- DOI: 10.1155/2015/936193
The role of innate immunity receptors in the pathogenesis of inflammatory bowel disease
Abstract
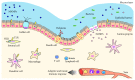
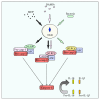
Innate immunity constitutes the first line of defense, fundamental for the recognition and the initiation of an inflammatory response against microorganisms. The innate immune response relies on the sensing of microbial-associated molecular patterns through specialized structures such as toll-like receptors (TLRs) and the nucleotide oligomerization domain- (NOD-) like receptors (NLRs). In the gut, these tasks are performed by the epithelial barrier and the presence of adaptive and innate immune mechanisms. TLRs and NLRs are distributed throughout the gastrointestinal mucosa, being more expressed in the epithelium, and in lamina propria immune and nonimmune cells. These innate immunity receptors exhibit complementary biological functions, with evidence for pathways overlapping. However, as tolerance is the predominant physiological response in the gastrointestinal mucosa, it appears that the TLRs are relatively downregulated, while NLRs play a critical role in mucosal defense in the gut. Over the past two decades, genetic polymorphisms have been associated with several diseases including inflammatory bowel disease. Special emphasis has been given to the susceptibility to Crohn's disease, in association with abnormalities in the NOD2 and in the NLRP3/inflammasome. Nevertheless, the mechanisms underlying innate immune receptors dysfunction that result in the persistent inflammation in inflammatory bowel disease remain to be clarified.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

