Further Stimulation of Cellular Immune Responses through Association of HPV-16 E6, E7 and L1 Genes in order to produce more Effective Therapeutic DNA Vaccines in Cervical Cancer Model
- PMID: 25821567
- PMCID: PMC4360347
Further Stimulation of Cellular Immune Responses through Association of HPV-16 E6, E7 and L1 Genes in order to produce more Effective Therapeutic DNA Vaccines in Cervical Cancer Model
Abstract
Background: Cervical cancer has been shown to be highly associated with human papillomavirus (HPV) infection. The viral oncogenes E6 and E7 are constantly expressed by the tumor cells and are therefore potent targets for therapeutic genetic vaccination. In the present study, it was investigated the potential effect of HPV-16 E6, E7 and L1 co-administration to activate specific cytotoxic T lymphocytes in tumor mice models.
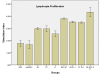
Methods: The HPV-16 E6, E7 and L1 genes from Iranian isolate were separately inserted into the mammalian expression vector, pcDNA3, to construct the DNA vaccine candidates. Tumor-bearing Animals (C57BL/6 mice) were immunized with the vaccine candidate; then, Lymphocyte Proliferation Assay (LPA) and relative tumor volume measurements were carried out in order to examine the immunological effects of the vaccine.
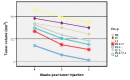
Results: Obtained results showed that co-administration of the HPV-16 E6, E7 and L1 DNA induced HPV-16 specific cellular immune responses and also protected against TC-1-induced tumor in vivo compared with negative controls.
Conclusion: The results showed that mixed delivery systems might be valuable to improve the magnitude of the induced immune responses and confirmed therapeutic effects of HPV-16 E6, E7 through cytotoxic T lymphocyte induction and illustrate the new promising role for HPV-16 L1 CTL epitopes as a suitable CTL inducer.
Keywords: immunocellular responses; pcDNA3/E6; pcDNA3/E7; pcDNA3/L1.
Figures


References
-
- Parkin DM. Global cancer statistics in the year 2000. Lancet oncol. 2001;2(9):533–43. - PubMed
-
- Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87(11):796–802. - PubMed
-
- Czegledy J, Rogo KO, Evander M, Wadell G. High-risk human papillomavirus types in cytologically normal cervical scrapes from Kenya. Med microbiol immunol. 1992;180(6):321–6. - PubMed
-
- Castellsagué X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol oncol. 2009;115(3):15–23. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
