Age-related alterations in the expression of genes and synaptic plasticity associated with nitric oxide signaling in the mouse dorsal striatum
- PMID: 25821602
- PMCID: PMC4364378
- DOI: 10.1155/2015/458123
Age-related alterations in the expression of genes and synaptic plasticity associated with nitric oxide signaling in the mouse dorsal striatum
Abstract
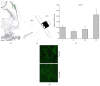
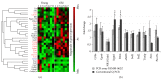
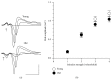
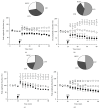
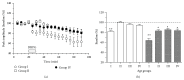
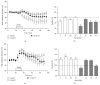
Age-related alterations in the expression of genes and corticostriatal synaptic plasticity were studied in the dorsal striatum of mice of four age groups from young (2-3 months old) to old (18-24 months of age) animals. A significant decrease in transcripts encoding neuronal nitric oxide (NO) synthase and receptors involved in its activation (NR1 subunit of the glutamate NMDA receptor and D1 dopamine receptor) was found in the striatum of old mice using gene array and real-time RT-PCR analysis. The old striatum showed also a significantly higher number of GFAP-expressing astrocytes and an increased expression of astroglial, inflammatory, and oxidative stress markers. Field potential recordings from striatal slices revealed age-related alterations in the magnitude and dynamics of electrically induced long-term depression (LTD) and significant enhancement of electrically induced long-term potentiation in the middle-aged striatum (6-7 and 12-13 months of age). Corticostriatal NO-dependent LTD induced by pharmacological activation of group I metabotropic glutamate receptors underwent significant reduction with aging and could be restored by inhibition of cGMP hydrolysis indicating that its age-related deficit is caused by an altered NO-cGMP signaling cascade. It is suggested that age-related alterations in corticostriatal synaptic plasticity may result from functional alterations in receptor-activated signaling cascades associated with increasing neuroinflammation and a prooxidant state.
Figures

References
-
- Langenecker S. A., Briceno E. M., Hamid N. M., Nielson K. A. An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: a study in the basal ganglia. Brain Research. 2007;1135(1):58–68. doi: 10.1016/j.brainres.2006.11.068. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

