Self-assembled anchor layers/polysaccharide coatings on titanium surfaces: a study of functionalization and stability
- PMID: 25821702
- PMCID: PMC4362089
- DOI: 10.3762/bjnano.6.63
Self-assembled anchor layers/polysaccharide coatings on titanium surfaces: a study of functionalization and stability
Abstract
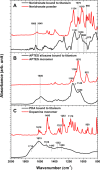
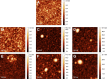
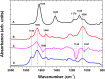
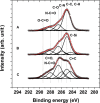
Composite materials based on a titanium support and a thin, alginate hydrogel could be used in bone tissue engineering as a scaffold material that provides biologically active molecules. The main objective of this contribution is to characterize the activation and the functionalization of titanium surfaces by the covalent immobilization of anchoring layers of self-assembled bisphosphonate neridronate monolayers and polymer films of 3-aminopropyltriethoxysilane and biomimetic poly(dopamine). These were further used to bind a bio-functional alginate coating. The success of the titanium surface activation, anchoring layer formation and alginate immobilization, as well as the stability upon immersion under physiological-like conditions, are demonstrated by different surface sensitive techniques such as spectroscopic ellipsometry, infrared reflection-absorption spectroscopy and X-ray photoelectron spectroscopy. The changes in morphology and the established continuity of the layers are examined by scanning electron microscopy, surface profilometry and atomic force microscopy. The changes in hydrophilicity after each modification step are further examined by contact angle goniometry.
Keywords: XPS; alginate; biomimetic surfaces; bisphosphonates; neridronate; poly(dopamine); spectroscopic ellipsometry; surface characterization; surface modification; titanium.
Figures




Similar articles
-
Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies.Polymers (Basel). 2021 Dec 31;14(1):165. doi: 10.3390/polym14010165. Polymers (Basel). 2021. PMID: 35012187 Free PMC article. Review.
-
Polysaccharide-protein surface modification of titanium via a layer-by-layer technique: characterization and cell behaviour aspects.Biomaterials. 2005 Oct;26(30):5960-71. doi: 10.1016/j.biomaterials.2005.03.020. Biomaterials. 2005. PMID: 15913761
-
A nitric oxide-catalytically generating carboxymethyl chitosan/sodium alginate hydrogel coating mimicking endothelium function for improving the biocompatibility.Int J Biol Macromol. 2023 Dec 31;253(Pt 1):126727. doi: 10.1016/j.ijbiomac.2023.126727. Epub 2023 Sep 9. Int J Biol Macromol. 2023. PMID: 37673159
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Characterization of Functional Materials Using Coherence Scanning Interferometry and Environmental Chambers.ACS Omega. 2023 Mar 14;8(12):10643-10655. doi: 10.1021/acsomega.2c07007. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008104 Free PMC article. Review.
Cited by
-
Antiadhesive and Antibacterial Coatings for Short-Term Titanium Implants.Macromol Rapid Commun. 2025 Jun;46(12):e2400989. doi: 10.1002/marc.202400989. Epub 2025 Mar 8. Macromol Rapid Commun. 2025. PMID: 40056084 Free PMC article.
-
Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies.Polymers (Basel). 2021 Dec 31;14(1):165. doi: 10.3390/polym14010165. Polymers (Basel). 2021. PMID: 35012187 Free PMC article. Review.
-
Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads.Int J Mol Sci. 2021 Oct 28;22(21):11666. doi: 10.3390/ijms222111666. Int J Mol Sci. 2021. PMID: 34769095 Free PMC article.
-
A New Insight into Coating's Formation Mechanism Between TiO2 and Alendronate on Titanium Dental Implant.Materials (Basel). 2020 Jul 20;13(14):3220. doi: 10.3390/ma13143220. Materials (Basel). 2020. PMID: 32698367 Free PMC article.
-
Interfacial Nanoengineering of Hydrogel Surfaces via Block Copolymer Self-Assembly.ACS Appl Mater Interfaces. 2025 Feb 12;17(6):10073-10086. doi: 10.1021/acsami.4c18632. Epub 2025 Feb 3. ACS Appl Mater Interfaces. 2025. PMID: 39901519 Free PMC article.
References
-
- Elias C N, Lima J H C, Valiev R, Meyers M A. JOM. 2008;60:46–49. doi: 10.1007/s11837-008-0031-1. - DOI
-
- Park J B, Bronzino J D, editors. Biomaterials - Principles and Applications. Boca Raton, FL, USA: CRC Press; 2003.
-
- Tanaka Y, Saito H, Tsutsumi Y, Doi H, Imai H, Hanawa T. Mater Trans. 2008;49:805–811. doi: 10.2320/matertrans.MRA2007317. - DOI
-
- Kataoka S, Gurau M C, Albertorio F, Holden M A, Lim S-M, Yang R D, Cremer P S. Langmuir. 2004;20:1662–1666. doi: 10.1021/la035971h. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
