Influence of gold, silver and gold-silver alloy nanoparticles on germ cell function and embryo development
- PMID: 25821705
- PMCID: PMC4362334
- DOI: 10.3762/bjnano.6.66
Influence of gold, silver and gold-silver alloy nanoparticles on germ cell function and embryo development
Abstract
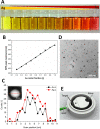
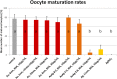
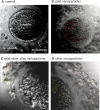
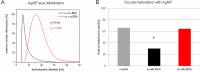
The use of engineered nanoparticles has risen exponentially over the last decade. Applications are manifold and include utilisation in industrial goods as well as medical and consumer products. Gold and silver nanoparticles play an important role in the current increase of nanoparticle usage. However, our understanding concerning possible side effects of this increased exposure to particles, which are frequently in the same size regime as medium sized biomolecules and accessorily possess highly active surfaces, is still incomplete. That particularly applies to reproductive aspects, were defects can be passed onto following generations. This review gives a brief overview of the most recent findings concerning reprotoxicological effects. The here presented data elucidate how composition, size and surface modification of nanoparticles influence viablility and functionality of reproduction relevant cells derived from various animal models. While in vitro cultured embryos displayed no toxic effects after the microinjection of gold and silver nanoparticles, sperm fertility parameters deteriorated after co-incubation with ligand free gold nanoparticles. However, the effect could be alleviated by bio-coating the nanoparticles, which even applies to silver and silver-rich alloy nanoparticles. The most sensitive test system appeared to be in vitro oocyte maturation showing a dose-dependent response towards protein (BSA) coated gold-silver alloy and silver nanoparticles leading up to complete arrest of maturation. Recent biodistribution studies confirmed that nanoparticles gain access to the ovaries and also penetrate the blood-testis and placental barrier. Thus, the design of nanoparticles with increased biosafety is highly relevant for biomedical applications.
Keywords: bimetallic nanoparticles, nano gold; nano silver; ontogenesis, oocyte; reprotoxicity; spermatozoa.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
