Molecular Imaging in Optical Coherence Tomography
- PMID: 25821718
- PMCID: PMC4373611
- DOI: 10.2174/2211555203666141117233442
Molecular Imaging in Optical Coherence Tomography
Abstract
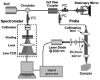
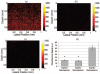
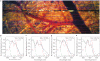
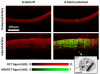
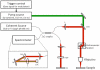
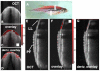
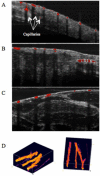
Optical coherence tomography (OCT) is a medical imaging technique that provides tomographic images at micron scales in three dimensions and high speeds. The addition of molecular contrast to the available morphological image holds great promise for extending OCT's impact in clinical practice and beyond. Fundamental limitations prevent OCT from directly taking advantage of powerful molecular processes such as fluorescence emission and incoherent Raman scattering. A wide range of approaches is being researched to provide molecular contrast to OCT. Here we review those approaches with particular attention to those that derive their molecular contrast directly from modulation of the OCT signal. We also provide a brief overview of the multimodal approaches to gaining molecular contrast coincident with OCT.
Figures


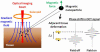







Similar articles
-
Spectral- and Polarization-Dependent Scattering of Gold Nanobipyramids for Exogenous Contrast in Optical Coherence Tomography.Nano Lett. 2021 Oct 27;21(20):8595-8601. doi: 10.1021/acs.nanolett.1c02291. Epub 2021 Oct 13. Nano Lett. 2021. PMID: 34644094 Free PMC article.
-
Co-registered combined OCT and THz imaging to extract depth and refractive index of a tissue-equivalent test object.Biomed Opt Express. 2020 Feb 19;11(3):1417-1431. doi: 10.1364/BOE.378506. eCollection 2020 Mar 1. Biomed Opt Express. 2020. PMID: 32206419 Free PMC article.
-
Application of optical coherence tomography to automated contact lens metrology.J Biomed Opt. 2010 Jan-Feb;15(1):016009. doi: 10.1117/1.3292598. J Biomed Opt. 2010. PMID: 20210455
-
Optical Coherence Tomography: Basic Concepts and Applications in Neuroscience Research.J Med Eng. 2017;2017:3409327. doi: 10.1155/2017/3409327. Epub 2017 Oct 29. J Med Eng. 2017. PMID: 29214158 Free PMC article. Review.
-
Methods and applications of full-field optical coherence tomography: a review.J Biomed Opt. 2022 May;27(5):050901. doi: 10.1117/1.JBO.27.5.050901. J Biomed Opt. 2022. PMID: 35596250 Free PMC article. Review.
Cited by
-
Photothermal optical coherence tomography for 3D live cell detection and mapping.Opt Contin. 2023 Dec 15;2(12):2468-2483. doi: 10.1364/optcon.503577. Epub 2023 Nov 27. Opt Contin. 2023. PMID: 38665863 Free PMC article.
-
Gold nanoparticles to enhance ophthalmic imaging.Biomater Sci. 2021 Jan 21;9(2):367-390. doi: 10.1039/d0bm01063d. Epub 2020 Oct 15. Biomater Sci. 2021. PMID: 33057463 Free PMC article. Review.
-
Gold nanomaterials for optical biosensing and bioimaging.Nanoscale Adv. 2021 Apr 14;3(10):2679-2698. doi: 10.1039/d0na00961j. eCollection 2021 May 18. Nanoscale Adv. 2021. PMID: 36134176 Free PMC article. Review.
-
Genetically Encodable Contrast Agents for Optical Coherence Tomography.ACS Nano. 2020 Jul 28;14(7):7823-7831. doi: 10.1021/acsnano.9b08432. Epub 2020 Feb 10. ACS Nano. 2020. PMID: 32023037 Free PMC article.
-
Renally Clearable Ultraminiature Chain-Like Gold Nanoparticle Clusters for Multimodal Molecular Imaging of Choroidal Neovascularization.Adv Mater. 2023 Aug;35(31):e2302069. doi: 10.1002/adma.202302069. Epub 2023 Jun 21. Adv Mater. 2023. PMID: 37285214 Free PMC article.
References
-
- Chen Y, Aguirre AD, Hsiung PL, Desai S, Herz PR, Pedrosa M, et al. Ultrahigh resolution optical coherence tomography of Barrett's esophagus: preliminary descriptive clinical study correlating images with histology. Endoscopy. 2007;39(7):599–605. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
